Neuronal Phenotype of col4a1 and col25a1: An Intriguing Hypothesis in Vertebrates Brain Aging
- PMID: 35163698
- PMCID: PMC8836537
- DOI: 10.3390/ijms23031778
Neuronal Phenotype of col4a1 and col25a1: An Intriguing Hypothesis in Vertebrates Brain Aging
Abstract
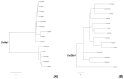
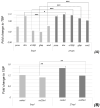
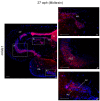
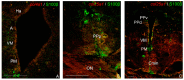
Collagens are the most abundant proteins in vertebrates and constitute the major components of the extracellular matrix. Collagens play an important and multifaceted role in the development and functioning of the nervous system and undergo structural remodeling and quantitative modifications during aging. Here, we investigated the age-dependent regulation of col4a1 and col25a1 in the brain of the short-lived vertebrate Nothobranchius furzeri, a powerful model organism for aging research due to its natural fast-aging process and further characterized typical hallmarks of brain aging in this species. We showed that col4a1 and col25a1 are relatively well conserved during vertebrate evolution, and their expression significantly increases in the brain of N. furzeri upon aging. Noteworthy, we report that both col4a1 and col25a1 are expressed in cells with a neuronal phenotype, unlike what has already been documented in mammalian brain, in which only col25a1 is considered a neuronal marker, whereas col4a1 seems to be expressed only in endothelial cells. Overall, our findings encourage further investigation on the role of col4a1 and col25a1 in the biology of the vertebrate brain as well as the onset of aging and neurodegenerative diseases.
Keywords: Nothobranchius furzeri; aging markers; central nervous system; collagens; fish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri.Aging Cell. 2011 Oct;10(5):824-31. doi: 10.1111/j.1474-9726.2011.00723.x. Epub 2011 Jun 27. Aging Cell. 2011. PMID: 21624037
-
A miRNA catalogue and ncRNA annotation of the short-living fish Nothobranchius furzeri.BMC Genomics. 2017 Sep 5;18(1):693. doi: 10.1186/s12864-017-3951-8. BMC Genomics. 2017. PMID: 28874118 Free PMC article.
-
RNA-seq of the aging brain in the short-lived fish N. furzeri - conserved pathways and novel genes associated with neurogenesis.Aging Cell. 2014 Dec;13(6):965-74. doi: 10.1111/acel.12257. Epub 2014 Jul 25. Aging Cell. 2014. PMID: 25059688 Free PMC article.
-
Annual fishes of the genus Nothobranchius as a model system for aging research.Aging Cell. 2005 Oct;4(5):223-33. doi: 10.1111/j.1474-9726.2005.00165.x. Aging Cell. 2005. PMID: 16164422 Review.
-
From the bush to the bench: the annual Nothobranchius fishes as a new model system in biology.Biol Rev Camb Philos Soc. 2016 May;91(2):511-33. doi: 10.1111/brv.12183. Epub 2015 Apr 28. Biol Rev Camb Philos Soc. 2016. PMID: 25923786 Review.
Cited by
-
Central and Peripheral NPY Age-Related Regulation: A Comparative Analysis in Fish Translational Models.Int J Mol Sci. 2022 Mar 30;23(7):3839. doi: 10.3390/ijms23073839. Int J Mol Sci. 2022. PMID: 35409198 Free PMC article.
-
Matrix Metalloproteinases in the Periodontium-Vital in Tissue Turnover and Unfortunate in Periodontitis.Int J Mol Sci. 2024 Feb 27;25(5):2763. doi: 10.3390/ijms25052763. Int J Mol Sci. 2024. PMID: 38474009 Free PMC article. Review.
-
Olfactory and gustatory chemical sensor systems in the African turquoise killifish: Insights from morphology.Cell Tissue Res. 2024 Dec;398(3):239-252. doi: 10.1007/s00441-024-03923-5. Epub 2024 Oct 21. Cell Tissue Res. 2024. PMID: 39432108 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

