Upregulation of Cathepsin X in Glioblastoma: Interplay with γ-Enolase and the Effects of Selective Cathepsin X Inhibitors
- PMID: 35163706
- PMCID: PMC8836869
- DOI: 10.3390/ijms23031784
Upregulation of Cathepsin X in Glioblastoma: Interplay with γ-Enolase and the Effects of Selective Cathepsin X Inhibitors
Abstract
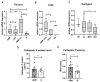
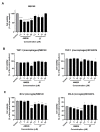
Glioblastoma (GBM) is the most common and deadly primary brain tumor in adults. Understanding GBM pathobiology and discovering novel therapeutic targets are critical to finding efficient treatments. Upregulation of the lysosomal cysteine carboxypeptidase cathepsin X has been linked to immune dysfunction and neurodegenerative diseases, but its role in cancer and particularly in GBM progression in patients is unknown. In this study, cathepsin X expression and activity were found to be upregulated in human GBM tissues compared to low-grade gliomas and nontumor brain tissues. Cathepsin X was localized in GBM cells as well as in tumor-associated macrophages and microglia. Subsequently, potent irreversible (AMS36) and reversible (Z7) selective cathepsin X inhibitors were tested in vitro. Selective cathepsin X inhibitors decreased the viability of patient-derived GBM cells as well as macrophages and microglia that were cultured in conditioned media of GBM cells. We next examined the expression pattern of neuron-specific enzyme γ-enolase, which is the target of cathepsin X. We found that there was a correlation between high proteolytic activity of cathepsin X and C-terminal cleavage of γ-enolase and that cathepsin X and γ-enolase were colocalized in GBM tissues, preferentially in GBM-associated macrophages and microglia. Taken together, our results on patient-derived material suggest that cathepsin X is involved in GBM progression and is a potential target for therapeutic approaches against GBM.
Keywords: cathepsin X; cathepsin X inhibitors; glioblastoma; glioblastoma stem cells; tumor microenvironment; γ-enolase.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Neuroprotective role of γ-enolase in microglia in a mouse model of Alzheimer's disease is regulated by cathepsin X.Aging Cell. 2013 Aug;12(4):604-14. doi: 10.1111/acel.12093. Epub 2013 May 27. Aging Cell. 2013. PMID: 23621429
-
The α- to γ-enolase switch: The role and regulation of γ-enolase during oligodendrocyte differentiation.Int J Biol Macromol. 2025 Apr;301:140464. doi: 10.1016/j.ijbiomac.2025.140464. Epub 2025 Jan 28. Int J Biol Macromol. 2025. PMID: 39884600
-
Programmed Cell Death 10 Mediated CXCL2-CXCR2 Signaling in Regulating Tumor-Associated Microglia/Macrophages Recruitment in Glioblastoma.Front Immunol. 2021 May 24;12:637053. doi: 10.3389/fimmu.2021.637053. eCollection 2021. Front Immunol. 2021. PMID: 34108959 Free PMC article.
-
Microglia in Cancer: For Good or for Bad?Adv Exp Med Biol. 2016;949:245-261. doi: 10.1007/978-3-319-40764-7_12. Adv Exp Med Biol. 2016. PMID: 27714693 Review.
-
Myeloid Cells in Glioblastoma Microenvironment.Cells. 2020 Dec 24;10(1):18. doi: 10.3390/cells10010018. Cells. 2020. PMID: 33374253 Free PMC article. Review.
Cited by
-
The multifaceted roles of cathepsins in immune and inflammatory responses: implications for cancer therapy, autoimmune diseases, and infectious diseases.Biomark Res. 2024 Dec 31;12(1):165. doi: 10.1186/s40364-024-00711-9. Biomark Res. 2024. PMID: 39736788 Free PMC article. Review.
-
The role of lysosomal peptidases in glioma immune escape: underlying mechanisms and therapeutic strategies.Front Immunol. 2023 Jun 16;14:1154146. doi: 10.3389/fimmu.2023.1154146. eCollection 2023. Front Immunol. 2023. PMID: 37398678 Free PMC article. Review.
-
Changes Induced by P2X7 Receptor Stimulation of Human Glioblastoma Stem Cells in the Proteome of Extracellular Vesicles Isolated from Their Secretome.Cells. 2024 Mar 25;13(7):571. doi: 10.3390/cells13070571. Cells. 2024. PMID: 38607010 Free PMC article.
-
Role of Glycolytic and Glutamine Metabolism Reprogramming on the Proliferation, Invasion, and Apoptosis Resistance through Modulation of Signaling Pathways in Glioblastoma.Int J Mol Sci. 2023 Dec 18;24(24):17633. doi: 10.3390/ijms242417633. Int J Mol Sci. 2023. PMID: 38139462 Free PMC article. Review.
-
Screening the Significant Hub Genes by Comparing Tumor Cells, Normoxic and Hypoxic Glioblastoma Stem-like Cell Lines Using Co-Expression Analysis in Glioblastoma.Genes (Basel). 2022 Mar 15;13(3):518. doi: 10.3390/genes13030518. Genes (Basel). 2022. PMID: 35328072 Free PMC article.
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Wen P.Y., Weller M., Lee E.Q., Alexander B.M., Barnholtz-Sloan J.S., Barthel F.P., Batchelor T.T., Bindra R.S., Chang S.M., Chiocca E.A., et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro. Oncol. 2020;22:1073–1113. doi: 10.1093/neuonc/noaa106. - DOI - PMC - PubMed
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

