Amniotic LPS-Induced Apoptosis in the Fetal Brain Is Suppressed by Vaginal LPS Preconditioning but Is Promoted by Continuous Ischemic Reperfusion
- PMID: 35163709
- PMCID: PMC8836254
- DOI: 10.3390/ijms23031787
Amniotic LPS-Induced Apoptosis in the Fetal Brain Is Suppressed by Vaginal LPS Preconditioning but Is Promoted by Continuous Ischemic Reperfusion
Abstract
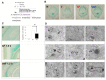
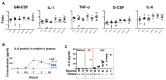
Chorioamnionitis (CAM) is an increasingly common disease affecting pregnant women which derives from bacterial vaginosis. In different clinical cases, it has been shown that CAM can cause multiple risk factors for fetal brain damage, such as infection, and intra-uterine asphyxia. However, the molecular mechanism remains unknown. In this study, we established a novel CAM mouse model by exposing pregnant mice to a combination of three risk factors: vaginal lipopolysaccharides (LPS), amniotic LPS, and ischemic reperfusion. We found amniotic LPS caused Parkinson's disease-like fetal brain damage, in a dose and time-dependent manner. Moreover, the mechanism of this fetal brain damage is apoptosis induced by amniotic LPS but it was inhibited by being pretreated with a vaginal LPS challenge before amniotic LPS injection. In contrast, amniotic LPS with continuous ischemic reperfusion caused a higher level of apoptotic cell death than amniotic LPS alone. In particular, a potential neuroprotective biomarker phosphorylation (p)-CREB (ser133) appeared in only vaginal LPS preconditioned before amniotic LPS, whereas ischemic reperfusion triggered IKK phosphorylation after amniotic LPS. Despite the need for many future investigations, this study also discussed a developed understanding of the molecular mechanism of how these phenotypes occurred.
Keywords: bacterial vaginosis; chorioamnionitis; fetal brain damage; mouse model; vaginal lipopolysaccharide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids.PLoS One. 2012;7(5):e38257. doi: 10.1371/journal.pone.0038257. Epub 2012 May 31. PLoS One. 2012. PMID: 22693607 Free PMC article.
-
Vaginal LPS changed gene transcriptional regulation response to ischemic reperfusion and increased vulnerability of fetal brain hemorrhage.Biochem Biophys Res Commun. 2015 Dec 4-11;468(1-2):228-33. doi: 10.1016/j.bbrc.2015.10.125. Epub 2015 Oct 30. Biochem Biophys Res Commun. 2015. PMID: 26523514
-
Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep.PLoS One. 2013 Dec 17;8(12):e81644. doi: 10.1371/journal.pone.0081644. eCollection 2013. PLoS One. 2013. PMID: 24358119 Free PMC article.
-
Chorioamnionitis - new ideas from experimental models.Neonatology. 2011;99(4):320-5. doi: 10.1159/000326620. Epub 2011 Jun 23. Neonatology. 2011. PMID: 21701204 Review.
-
The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity--a review.Arch Gynecol Obstet. 1990;247(1):1-13. doi: 10.1007/BF02390649. Arch Gynecol Obstet. 1990. PMID: 2178562 Review.
Cited by
-
Therapeutic and Prophylactic Effects of Amphotericin B Liposomes on Chronic Social Defeat Stress-Induced Behavioral Abnormalities in Mice.Front Pharmacol. 2022 Jul 15;13:918177. doi: 10.3389/fphar.2022.918177. eCollection 2022. Front Pharmacol. 2022. PMID: 35910388 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

