Lipid Droplet Accumulation Promotes RPE Dysfunction
- PMID: 35163712
- PMCID: PMC8836556
- DOI: 10.3390/ijms23031790
Lipid Droplet Accumulation Promotes RPE Dysfunction
Abstract
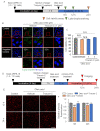
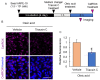
Non-exudative age-related macular degeneration (AMD) is an irreversibly progressive retinal degenerative disease characterized by dysfunction and loss of retinal pigment epithelium (RPE). It has been suggested that impaired phagocytosis of the RPE is involved in the progression of non-exudative AMD, but the mechanism is not fully clear. In this study, we investigated the effect of lipid droplet accumulation on RPE function. Compared to young mice, the expression of lipid droplet-associated proteins increased in the RPE-choroidal complex, and lipid droplet in the RPE was observed in aged pigmented mice (12-month-old). Repeated treatment of the photoreceptor outer segment against ARPE-19 resulted in lipid droplets in ARPE-19 cells in vitro. Oleic acid treatment for ARPE-19 cells to form intracellular lipid droplet reduced the POS uptake into the ARPE-19 cells without causing a decrease in cell viability. The suppression of the POS uptake by lipid droplet formation improved by inhibiting lipid droplet formation using triacsin C. Moreover, the amount of intracellular reactive oxygen species was suppressed by the triacsin C treatment. These results indicate that lipid droplet is involved in the RPE dysfunction, and inhibiting lipid droplet formation may be a target for preventing and treating non-exudative AMD.
Keywords: aging; lipid droplet; phagocytosis; retinal pigment epithelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yao J., Jia L., Khan N., Lin C., Mitter S.K., Boulton M.E., Dunaief J.L., Klionsky D.J., Guan J.-L., Thompson D.A., et al. Deletion of Autophagy Inducer RB1CC1 Results in Degeneration of the Retinal Pigment Epithelium. Autophagy. 2015;11:939–953. doi: 10.1080/15548627.2015.1041699. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

