Reversible Redox Processes in Polymer of Unmetalated Salen-Type Ligand: Combined Electrochemical in Situ Studies and Direct Comparison with Corresponding Nickel Metallopolymer
- PMID: 35163715
- PMCID: PMC8836782
- DOI: 10.3390/ijms23031795
Reversible Redox Processes in Polymer of Unmetalated Salen-Type Ligand: Combined Electrochemical in Situ Studies and Direct Comparison with Corresponding Nickel Metallopolymer
Abstract
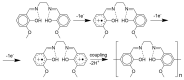
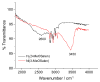
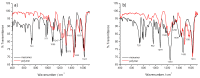
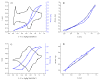
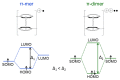
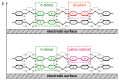
Most non-metalized Salen-type ligands form passivation thin films on electrode surfaces upon electrochemical oxidation. In contrast, the H2(3-MeOSalen) forms electroactive polymer films similarly to the corresponding nickel complex. There are no details of electrochemistry, doping mechanism and charge transfer pathways in the polymers of pristine Salen-type ligands. We studied a previously uncharacterized electrochemically active polymer of a Salen-type ligand H2(3-MeOSalen) by a combination of cyclic voltammetry, in situ ultraviolet-visible (UV-VIS) spectroelectrochemistry, in situ electrochemical quartz crystal microbalance and Fourier Transform infrared spectroscopy (FTIR) spectroscopy. By directly comparing it with the polymer of a Salen-type nickel complex poly-Ni(3-MeOSalen) we elucidate the effect of the central metal atom on the structure and charge transport properties of the electrochemically doped polymer films. We have shown that the mechanism of charge transfer in the polymeric ligand poly-H2(3-MeOSalen) are markedly different from the corresponding polymeric nickel complex. Due to deviation from planarity of N2O2 sphere for the ligand H2(3-MeOSalen), the main pathway of electron transfer in the polymer film poly-H2(3-MeOSalen) is between π-stacked structures (the π-electronic systems of phenyl rings are packed face-to-face) and C-C bonded phenyl rings. The main way of electron transfer in the polymer film poly-Ni(3-MeOSalen) is along the polymer chain, while redox processes are ligand-based.
Keywords: Schiff bases; UV–vis–NIR spectroelectrochemistry; conducting polymer; electrochemical polymerization; salen-type ligand; π-stacked structures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





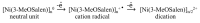

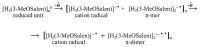
Similar articles
-
Nickel(II) Complex of N4 Schiff Base Ligand as a Building Block for a Conducting Metallopolymer with Multiple Redox States.Molecules. 2021 Apr 30;26(9):2646. doi: 10.3390/molecules26092646. Molecules. 2021. PMID: 33946577 Free PMC article.
-
Influence of Electron-Withdrawing Substituents on the Electronic Structure of Oxidized Ni and Cu Salen Complexes.Inorg Chem. 2015 Jun 15;54(12):5970-80. doi: 10.1021/acs.inorgchem.5b00783. Epub 2015 May 27. Inorg Chem. 2015. PMID: 26016716
-
The Influence of Electrolyte Type on Kinetics of Redox Processes in the Polymer Films of Ni(II) Salen-Type Complexes.Molecules. 2022 Mar 10;27(6):1812. doi: 10.3390/molecules27061812. Molecules. 2022. PMID: 35335175 Free PMC article.
-
Spectroelectrochemistry of Electroactive Polymer Composite Materials.Polymers (Basel). 2022 Aug 5;14(15):3201. doi: 10.3390/polym14153201. Polymers (Basel). 2022. PMID: 35956715 Free PMC article. Review.
-
A modular, energy-based approach to the development of nickel containing molecular electrocatalysts for hydrogen production and oxidation.Biochim Biophys Acta. 2013 Aug-Sep;1827(8-9):1123-39. doi: 10.1016/j.bbabio.2013.01.003. Epub 2013 Jan 11. Biochim Biophys Acta. 2013. PMID: 23313415 Review.
Cited by
-
Implementing the donor-acceptor approach in electronically conducting copolymers via electropolymerization.RSC Adv. 2022 Apr 20;12(19):12089-12115. doi: 10.1039/d2ra01176j. eCollection 2022 Apr 13. RSC Adv. 2022. PMID: 35481093 Free PMC article. Review.
References
-
- Zhan C., Yu G., Lu Y., Wang L., Wujcik E., Wei S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C. 2017;5:1569–1585. doi: 10.1039/C6TC04269D. - DOI
-
- Guo P., Hui T.-W., Cheung K.-C., Wong K.-Y., Shiu K.-K. Charge propagation in nickel 6,6′-bis(2′-hydroxyphenyl)-2,2′-bipyridine polymer film doped with perchlorate anions. J. Electroanal. Chem. 2001;498:142–151. doi: 10.1016/S0022-0728(00)00347-8. - DOI
-
- Rudge A., Raistrick I., Gottesfeld S., Ferraris J.P. A study of the electrochemical properties of conducting polymers for application in electrochemical capacitors. Electrochimica Acta. 1994;39:273–287. doi: 10.1016/0013-4686(94)80063-4. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

