Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice
- PMID: 35163729
- PMCID: PMC8836558
- DOI: 10.3390/ijms23031807
Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice
Abstract
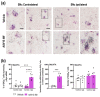
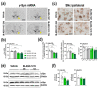
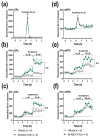
The synuclein family consists of α-, β-, and γ-Synuclein (α-Syn, β-Syn, and γ-Syn) expressed in the neurons and concentrated in synaptic terminals. While α-Syn is at the center of interest due to its implication in the pathogenesis of Parkinson's disease (PD) and other synucleinopathies, limited information exists on the other members. The current study aimed at investigating the biological role of γ-Syn controlling the midbrain dopamine (DA) function. We generated two different mouse models with: (i) γ-Syn overexpression induced by an adeno-associated viral vector and (ii) γ-Syn knockdown induced by a ligand-conjugated antisense oligonucleotide, in order to modify the endogenous γ-Syn transcription levels in midbrain DA neurons. The progressive overexpression of γ-Syn decreased DA neurotransmission in the nigrostriatal and mesocortical pathways. In parallel, mice evoked motor deficits in the rotarod and impaired cognitive performance as assessed by novel object recognition, passive avoidance, and Morris water maze tests. Conversely, acute γ-Syn knockdown selectively in DA neurons facilitated forebrain DA neurotransmission. Importantly, modifications in γ-Syn expression did not induce the loss of DA neurons or changes in α-Syn expression. Collectively, our data strongly suggest that DA release/re-uptake processes in the nigrostriatal and mesocortical pathways are partially dependent on substantia nigra pars compacta /ventral tegmental area (SNc/VTA) γ-Syn transcription levels, and are linked to modulation of DA transporter function, similar to α-Syn.
Keywords: AAV vector; antisense oligonucleotide; cognitive dysfunction; dopamine; motor deficits; γ-synuclein.
Conflict of interest statement
A.B. is an inventor of the issued patents WO2011131693, WO-2014064257-A1, WO2014064258-A1 for ligand-conjugated siRNA and ASO molecules and the targeting approach for monoamine systems related to this work. The rest of the authors declare no competing interest.
Figures



References
-
- Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Verdugo J.M., Armanini M., Ryan A., et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

