Tumor-Associated Macrophages/Microglia in Glioblastoma Oncolytic Virotherapy: A Double-Edged Sword
- PMID: 35163730
- PMCID: PMC8836356
- DOI: 10.3390/ijms23031808
Tumor-Associated Macrophages/Microglia in Glioblastoma Oncolytic Virotherapy: A Double-Edged Sword
Abstract
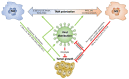
Oncolytic virotherapy is a rapidly progressing field that uses oncolytic viruses (OVs) to selectively infect malignant cells and cause an antitumor response through direct oncolysis and stimulation of the immune system. Despite demonstrated pre-clinical efficacy of OVs in many cancer types and some favorable clinical results in glioblastoma (GBM) trials, durable increases in overall survival have remained elusive. Recent evidence has emerged that tumor-associated macrophage/microglia (TAM) involvement is likely an important factor contributing to OV treatment failure. It is prudent to note that the relationship between TAMs and OV therapy failures is complex. Canonically activated TAMs (i.e., M1) drive an antitumor response while also inhibiting OV replication and spread. Meanwhile, M2 activated TAMs facilitate an immunosuppressive microenvironment thereby indirectly promoting tumor growth. In this focused review, we discuss the complicated interplay between TAMs and OV therapies in GBM. We review past studies that aimed to maximize effectiveness through immune system modulation-both immunostimulatory and immunosuppressant-and suggest future directions to maximize OV efficacy.
Keywords: glioblastoma (GBM); oncolytic virotherapy; tumor microenvironment; tumor-associated macrophages/microglia (TAMs).
Conflict of interest statement
J.D.B. has an equity position in Avidea Technologies, Inc., which is commercializing polymer-based drug delivery technologies for immunotherapeutic applications and has an equity position in Treovir LLC, an oHSV clinical stage company and is a member of the POCKiT Diagnostics Board of Scientific Advisors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Oncolytic Virotherapy Blockade by Microglia and Macrophages Requires STAT1/3.Cancer Res. 2018 Feb 1;78(3):718-730. doi: 10.1158/0008-5472.CAN-17-0599. Epub 2017 Nov 8. Cancer Res. 2018. PMID: 29118089
-
Enhancing oncolytic virotherapy by extracellular vesicle mediated microRNA reprograming of the tumour microenvironment.Front Immunol. 2024 Dec 23;15:1500570. doi: 10.3389/fimmu.2024.1500570. eCollection 2024. Front Immunol. 2024. PMID: 39763667 Free PMC article.
-
The Multifaceted Role of Macrophages in Oncolytic Virotherapy.Viruses. 2021 Aug 9;13(8):1570. doi: 10.3390/v13081570. Viruses. 2021. PMID: 34452439 Free PMC article. Review.
-
The Impact of Macrophage- and Microglia-Secreted TNFα on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment.Clin Cancer Res. 2015 Jul 15;21(14):3274-85. doi: 10.1158/1078-0432.CCR-14-3118. Epub 2015 Mar 31. Clin Cancer Res. 2015. PMID: 25829396 Free PMC article.
-
Overcoming Barriers in Oncolytic Virotherapy with HDAC Inhibitors and Immune Checkpoint Blockade.Viruses. 2016 Jan 6;8(1):9. doi: 10.3390/v8010009. Viruses. 2016. PMID: 26751469 Free PMC article. Review.
Cited by
-
Interactions between microglia and glioma in tumor microenvironment.Front Oncol. 2023 Aug 28;13:1236268. doi: 10.3389/fonc.2023.1236268. eCollection 2023. Front Oncol. 2023. PMID: 37700840 Free PMC article. Review.
-
Engineered oncolytic virus coated with anti-PD-1 and alendronate for ameliorating intratumoral T cell hypofunction.Exp Hematol Oncol. 2025 Feb 15;14(1):16. doi: 10.1186/s40164-025-00611-0. Exp Hematol Oncol. 2025. PMID: 39955603 Free PMC article.
-
Microglia and Brain Macrophages as Drivers of Glioma Progression.Int J Mol Sci. 2022 Dec 9;23(24):15612. doi: 10.3390/ijms232415612. Int J Mol Sci. 2022. PMID: 36555253 Free PMC article. Review.
-
Emerging Biomarkers for Immunotherapy in Glioblastoma.Cancers (Basel). 2022 Apr 12;14(8):1940. doi: 10.3390/cancers14081940. Cancers (Basel). 2022. PMID: 35454848 Free PMC article. Review.
-
Why Don't the Mutant Cells That Evade DNA Repair Cause Cancer More Frequently? Importance of the Innate Immune System in the Tumor Microenvironment.Int J Mol Sci. 2023 Mar 6;24(5):5026. doi: 10.3390/ijms24055026. Int J Mol Sci. 2023. PMID: 36902456 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

