Interaction of Alpha Synuclein and Microtubule Organization Is Linked to Impaired Neuritic Integrity in Parkinson's Patient-Derived Neuronal Cells
- PMID: 35163733
- PMCID: PMC8836605
- DOI: 10.3390/ijms23031812
Interaction of Alpha Synuclein and Microtubule Organization Is Linked to Impaired Neuritic Integrity in Parkinson's Patient-Derived Neuronal Cells
Abstract
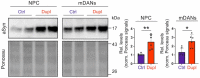
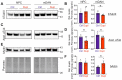
Parkinson's disease (PD) is neuropathologically characterized by the loss of dopaminergic neurons and the deposition of aggregated alpha synuclein (aSyn). Mounting evidence suggests that neuritic degeneration precedes neuronal loss in PD. A possible underlying mechanism could be the interference of aSyn with microtubule organization in the neuritic development, as implied by several studies using cell-free model systems. In this study, we investigate the impact of aSyn on microtubule organization in aSyn overexpressing H4 neuroglioma cells and midbrain dopaminergic neuronal cells (mDANs) generated from PD patient-derived human induced pluripotent stem cells (hiPSCs) carrying an aSyn gene duplication (SNCADupl). An unbiased mass spectrometric analysis reveals a preferential binding of aggregated aSyn conformers to a number of microtubule elements. We confirm the interaction of aSyn with beta tubulin III in H4 and hiPSC-derived mDAN cell model systems, and demonstrate a remarkable redistribution of tubulin isoforms from the soluble to insoluble fraction, accompanied by a significantly increased insoluble aSyn level. Concordantly, SNCADupl mDANs show impaired neuritic phenotypes characterized by perturbations in neurite initiation and outgrowth. In summary, our findings suggest a mechanistic pathway, through which aSyn aggregation interferes with microtubule organization and induces neurite impairments.
Keywords: Parkinson’s disease; SNCA duplication; alpha-synuclein; iPSC; microtubule; neurite; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Shahmoradian S.H., Lewis A.J., Genoud C., Hench J., Moors T.E., Navarro P.P., Castano-Diez D., Schweighauser G., Graff-Meyer A., Goldie K.N., et al. Lewy Pathology in Parkinson’s Disease Consists of Crowded Organelles and Lipid Membranes. Nat. Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- advanced project E30/the Interdisciplinary Center for Clinical Research of the University Hospital Erlangen, Germany
- 270949263/GRK2162, WI 3567/2-1, 125440785/SFB877 project B11 and Z2/Deutsche Forschungsgemeinschaft
- ForInter/Bavarian Research Consortium "Interaction of Human Brain Cells" (ForInter)
- 10.19.2.024MN to IP/Fritz Thyssen Foundation
- 01EK1605B/the Bundesministerium für Bildung und Forschung, Germany (BMBF, HitTau)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

