The Role of Oncostatin M and Its Receptor Complexes in Cardiomyocyte Protection, Regeneration, and Failure
- PMID: 35163735
- PMCID: PMC8836542
- DOI: 10.3390/ijms23031811
The Role of Oncostatin M and Its Receptor Complexes in Cardiomyocyte Protection, Regeneration, and Failure
Abstract
Oncostatin M (OSM), a member of the interleukin-6 family, functions as a major mediator of cardiomyocyte remodeling under pathological conditions. Its involvement in a variety of human cardiac diseases such as aortic stenosis, myocardial infarction, myocarditis, cardiac sarcoidosis, and various cardiomyopathies make the OSM receptor (OSMR) signaling cascades a promising therapeutic target. However, the development of pharmacological treatment strategies is highly challenging for many reasons. In mouse models of heart disease, OSM elicits opposing effects via activation of the type II receptor complex (OSMR/gp130). Short-term activation of OSMR/gp130 protects the heart after acute injury, whereas chronic activation promotes the development of heart failure. Furthermore, OSM has the ability to integrate signals from unrelated receptors that enhance fetal remodeling (dedifferentiation) of adult cardiomyocytes. Because OSM strongly stimulates the production and secretion of extracellular proteins, it is likely to exert systemic effects, which in turn, could influence cardiac remodeling. Compared with the mouse, the complexity of OSM signaling is even greater in humans because this cytokine also activates the type I leukemia inhibitory factor receptor complex (LIFR/gp130). In this article, we provide an overview of OSM-induced cardiomyocyte remodeling and discuss the consequences of OSMR/gp130 and LIFR/gp130 activation under acute and chronic conditions.
Keywords: cardiomyocyte; dedifferentiation; gp130; heart failure; inflammation; interleukin-6; leukemia inhibitory factor receptor; myocardial infarction; oncostatin M receptor; remodeling.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures




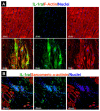
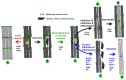
References
-
- Hou Y., Adrian-Segarra J.M., Richter M., Kubin N., Shin J., Werner I., Walther T., Schönburg M., Pöling J., Warnecke H., et al. Animal Models and “Omics” Technologies for Identification of Novel Biomarkers and Drug Targets to Prevent Heart Failure. BioMed Res. Int. 2015;2015:212910. doi: 10.1155/2015/212910. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

