Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins
- PMID: 35163748
- PMCID: PMC8836588
- DOI: 10.3390/ijms23031818
Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins
Abstract
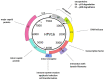
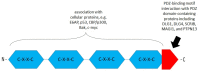
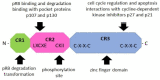
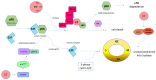
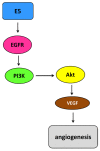
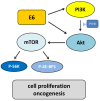
Human papillomaviruses (HPVs), which belong to the Papillomaviridae family, constitute a group of small nonenveloped double-stranded DNA viruses. HPV has a small genome that only encodes a few proteins, and it is also responsible for 5% of all human cancers, including cervical, vaginal, vulvar, penile, anal, and oropharyngeal cancers. HPV types may be classified as high- and low-risk genotypes (HR-HPVs and LR-HPVs, respectively) according to their oncogenic potential. HR-HPV 16 and 18 are the most common types worldwide and are the primary types that are responsible for most HPV-related cancers. The activity of the viral E6 and E7 oncoproteins, which interfere with critical cell cycle points such as suppressive tumor protein p53 (p53) and retinoblastoma protein (pRB), is the major contributor to HPV-induced neoplastic initiation and progression of carcinogenesis. In addition, the E5 protein might also play a significant role in tumorigenesis. The role of HPV in the pathogenesis of gynecological cancers is still not fully understood, which indicates a wide spectrum of potential research areas. This review focuses on HPV biology, the distribution of HPVs in gynecological cancers, the properties of viral oncoproteins, and the molecular mechanisms of carcinogenesis.
Keywords: gynecological cancers; human papillomavirus; oncoprotein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bosch F.X., Broker T.R., Forman D., Moscicki A.B., Gillison M.L., Doorbar J., Stern P.L., Stanley M., Arbyn M., Poljak M., et al. ICO Monograph ‘Comprehensive Control of HPV Infections and Related Diseases’. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31:I1–I31. doi: 10.1016/j.vaccine.2013.07.026. - DOI - PMC - PubMed
-
- Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V., Snijders P.J., Peto J., Meijer C.J., Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
-
- de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B., Tous S., Felix A., Bravo L.E., Shin H.R., et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. - DOI - PubMed
-
- Bilyk O.O., Pande N.T., Pejovic T., Buchynska L.G. The frequency of Human Papillomavirus types 16, 18 in upper genital tract of women at high risk of developing ovarian cancer. Exp. Oncol. 2014;36:121–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

