General Anesthesia and the Young Brain: The Importance of Novel Strategies with Alternate Mechanisms of Action
- PMID: 35163810
- PMCID: PMC8836828
- DOI: 10.3390/ijms23031889
General Anesthesia and the Young Brain: The Importance of Novel Strategies with Alternate Mechanisms of Action
Abstract
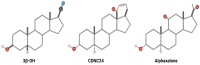
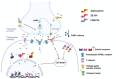
Over the past three decades, we have been grappling with rapidly accumulating evidence that general anesthetics (GAs) may not be as innocuous for the young brain as we previously believed. The growing realization comes from hundreds of animal studies in numerous species, from nematodes to higher mammals. These studies argue that early exposure to commonly used GAs causes widespread apoptotic neurodegeneration in brain regions critical to cognition and socio-emotional development, kills a substantial number of neurons in the young brain, and, importantly, results in lasting disturbances in neuronal synaptic communication within the remaining neuronal networks. Notably, these outcomes are often associated with long-term impairments in multiple cognitive-affective domains. Not only do preclinical studies clearly demonstrate GA-induced neurotoxicity when the exposures occur in early life, but there is a growing body of clinical literature reporting similar cognitive-affective abnormalities in young children who require GAs. The need to consider alternative GAs led us to focus on synthetic neuroactive steroid analogues that have emerged as effective hypnotics, and analgesics that are apparently devoid of neurotoxic effects and long-term cognitive impairments. This would suggest that certain steroid analogues with different cellular targets and mechanisms of action may be safe alternatives to currently used GAs. Herein we summarize our current knowledge of neuroactive steroids as promising novel GAs.
Keywords: general anesthetics; neuroactive steroid analogues; neurotoxicity; synaptogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
General anesthetics and cytotoxicity: possible implications for brain health.Drug Chem Toxicol. 2017 Apr;40(2):241-249. doi: 10.1080/01480545.2016.1188306. Epub 2016 Jun 2. Drug Chem Toxicol. 2017. PMID: 27252089 Review.
-
An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function.Anesth Analg. 2008 Jun;106(6):1681-707. doi: 10.1213/ane.0b013e318167ad77. Anesth Analg. 2008. PMID: 18499597 Review.
-
Early exposure to general anaesthesia and increasing trends in developmental behavioural impairments: is there a link?Br J Anaesth. 2023 Aug;131(2):208-211. doi: 10.1016/j.bja.2023.04.005. Epub 2023 May 10. Br J Anaesth. 2023. PMID: 37173202
-
Neurotoxicity of general anesthetics: an update.Curr Pharm Des. 2012;18(38):6232-40. doi: 10.2174/138161212803832344. Curr Pharm Des. 2012. PMID: 22762477 Review.
-
Developmental synaptogenesis and general anesthesia: a kiss of death?Curr Pharm Des. 2012;18(38):6225-31. doi: 10.2174/138161212803832380. Curr Pharm Des. 2012. PMID: 22762476 Review.
Cited by
-
From neurotoxicity to neuroprotection: Rethinking GABAAR-targeting anesthetics.Cell Biol Toxicol. 2025 Jun 14;41(1):104. doi: 10.1007/s10565-025-10057-z. Cell Biol Toxicol. 2025. PMID: 40516005 Free PMC article. Review.
-
Unveiling the hidden dangers: a review of non-apoptotic programmed cell death in anesthetic-induced developmental neurotoxicity.Cell Biol Toxicol. 2024 Aug 2;40(1):63. doi: 10.1007/s10565-024-09895-0. Cell Biol Toxicol. 2024. PMID: 39093513 Free PMC article. Review.
-
Malignant Brain Aging: The Formidable Link Between Dysregulated Signaling Through Mechanistic Target of Rapamycin Pathways and Alzheimer's Disease (Type 3 Diabetes).J Alzheimers Dis. 2023;95(4):1301-1337. doi: 10.3233/JAD-230555. J Alzheimers Dis. 2023. PMID: 37718817 Free PMC article. Review.
-
The Role of GABA Receptors in Anesthesia and Sedation: An Updated Review.CNS Drugs. 2025 Jan;39(1):39-54. doi: 10.1007/s40263-024-01128-6. Epub 2024 Oct 27. CNS Drugs. 2025. PMID: 39465449 Free PMC article. Review.
-
Propofol attenuates kinesin-mediated axonal vesicle transport and fusion.Mol Biol Cell. 2022 Nov 1;33(13):ar119. doi: 10.1091/mbc.E22-07-0276. Epub 2022 Sep 14. Mol Biol Cell. 2022. PMID: 36103253 Free PMC article.
References
-
- Jevtovic-Todorovic V., Hartman R.E., Izumi Y., Benshoff N.D., Dikranian K., Zorumski C.F., Olney J.W., Wozniak D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. - DOI - PMC - PubMed
-
- Young C., Jevtovic-Todorovic V., Qin Y.Q., Tenkova T., Wang H., Labruyere J., Olney J.W. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br. J. Pharm. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD097990/HD/NICHD NIH HHS/United States
- R35 GM141802/GM/NIGMS NIH HHS/United States
- R01 GM123746; R35 GM141802; R01 HD097990; R01 HD044517; R01 HD044517-S; R01 GM118197; R01 GM118197-S1./NH/NIH HHS/United States
- N/A/*Supported in part by funds from the Department of Anesthesiology at the University of Colorado Anschutz Medical campus, CU Medicine Endowment (to V.J-T.) and funds from the Taylor Family Institute for Innovative Psychiatric Research (to D.F.C.)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

