Oxidative DNA Damage and Cisplatin Neurotoxicity Is Exacerbated by Inhibition of OGG1 Glycosylase Activity and APE1 Endonuclease Activity in Sensory Neurons
- PMID: 35163831
- PMCID: PMC8836551
- DOI: 10.3390/ijms23031909
Oxidative DNA Damage and Cisplatin Neurotoxicity Is Exacerbated by Inhibition of OGG1 Glycosylase Activity and APE1 Endonuclease Activity in Sensory Neurons
Abstract
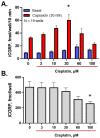
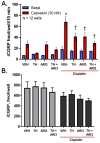
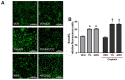
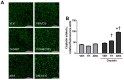
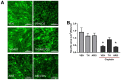
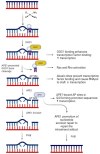
Cisplatin can induce peripheral neuropathy, which is a common complication of anti-cancer treatment and negatively impacts cancer survivors during and after completion of treatment; therefore, the mechanisms by which cisplatin alters sensory neuronal function to elicit neuropathy are the subject of much investigation. Our previous work suggests that the DNA repair activity of APE1/Ref-1, the rate-limiting enzyme of the base excision repair (BER) pathway, is critical for neuroprotection against cisplatin. A specific role for 8-oxoguanine DNA glycosylase-1 (OGG1), the glycosylase that removes the most common oxidative DNA lesion, and putative coordination of OGG1 with APE1/Ref-1 in sensory neurons, has not been investigated. We investigated whether inhibiting OGG1 glycosylase activity with the small molecule inhibitor, TH5487, and/or APE1/Ref-1 endonuclease activity with APE Repair Inhibitor III would alter the neurotoxic effects of cisplatin in sensory neuronal cultures. Sensory neuron function was assessed by calcitonin gene-related peptide (CGRP) release, as a marker of sensitivity and by neurite outgrowth. Cisplatin altered neuropeptide release in an inverse U-shaped fashion, with low concentrations enhancing and higher concentrations diminishing CGRP release. Pretreatment with BER inhibitors exacerbated the functional effects of cisplatin and enhanced 8oxo-dG and adduct lesions in the presence of cisplatin. Our studies demonstrate that inhibition of OGG1 and APE1 endonuclease activity enhances oxidative DNA damage and exacerbates neurotoxicity, thus limiting oxidative DNA damage in sensory neurons that might alleviate cisplatin-induced neuropathy.
Keywords: DNA damage; base excision repair; chemotherapy-induced peripheral neuropathy; cisplatin; neurite outgrowth; neuropeptide; oxidative stress; sensory neuron.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy.PLoS One. 2014 Sep 4;9(9):e106485. doi: 10.1371/journal.pone.0106485. eCollection 2014. PLoS One. 2014. PMID: 25188410 Free PMC article.
-
Small molecule activation of apurinic/apyrimidinic endonuclease 1 reduces DNA damage induced by cisplatin in cultured sensory neurons.DNA Repair (Amst). 2016 May;41:32-41. doi: 10.1016/j.dnarep.2016.03.009. Epub 2016 Mar 29. DNA Repair (Amst). 2016. PMID: 27078577 Free PMC article.
-
The role of the N-terminal domain of human apurinic/apyrimidinic endonuclease 1, APE1, in DNA glycosylase stimulation.DNA Repair (Amst). 2018 Apr;64:10-25. doi: 10.1016/j.dnarep.2018.02.001. Epub 2018 Feb 11. DNA Repair (Amst). 2018. PMID: 29475157
-
UV-DDB as a General Sensor of DNA Damage in Chromatin: Multifaceted Approaches to Assess Its Direct Role in Base Excision Repair.Int J Mol Sci. 2023 Jun 15;24(12):10168. doi: 10.3390/ijms241210168. Int J Mol Sci. 2023. PMID: 37373320 Free PMC article. Review.
-
The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target.Mol Aspects Med. 2007 Jun-Aug;28(3-4):375-95. doi: 10.1016/j.mam.2007.04.005. Epub 2007 May 3. Mol Aspects Med. 2007. PMID: 17560642 Review.
Cited by
-
Bis-Cinnamamide Derivatives as APE/Ref-1 Inhibitors for the Treatment of Human Melanoma.Molecules. 2022 Apr 21;27(9):2672. doi: 10.3390/molecules27092672. Molecules. 2022. PMID: 35566022 Free PMC article.
-
Molecular and Cellular Involvement in CIPN.Biomedicines. 2024 Mar 28;12(4):751. doi: 10.3390/biomedicines12040751. Biomedicines. 2024. PMID: 38672107 Free PMC article. Review.
-
Emerging and established therapies for chemotherapy-induced ototoxicity.J Cancer Surviv. 2023 Feb;17(1):17-26. doi: 10.1007/s11764-022-01317-6. Epub 2023 Jan 13. J Cancer Surviv. 2023. PMID: 36637631 Review.
-
APE1 condensation in nucleoli of non-cancer cells depends on rRNA transcription and forming G-quadruplex RNA structures.Nucleic Acids Res. 2025 Feb 27;53(5):gkaf168. doi: 10.1093/nar/gkaf168. Nucleic Acids Res. 2025. PMID: 40103231 Free PMC article.
-
Carbon Monoxide-Loaded Red Blood Cell Prevents the Onset of Cisplatin-Induced Acute Kidney Injury.Antioxidants (Basel). 2023 Sep 1;12(9):1705. doi: 10.3390/antiox12091705. Antioxidants (Basel). 2023. PMID: 37760008 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

