Why Monoamine Oxidase B Preferably Metabolizes N-Methylhistamine over Histamine: Evidence from the Multiscale Simulation of the Rate-Limiting Step
- PMID: 35163835
- PMCID: PMC8836602
- DOI: 10.3390/ijms23031910
Why Monoamine Oxidase B Preferably Metabolizes N-Methylhistamine over Histamine: Evidence from the Multiscale Simulation of the Rate-Limiting Step
Abstract
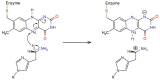
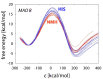
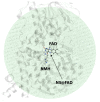
Histamine levels in the human brain are controlled by rather peculiar metabolic pathways. In the first step, histamine is enzymatically methylated at its imidazole Nτ atom, and the produced N-methylhistamine undergoes an oxidative deamination catalyzed by monoamine oxidase B (MAO-B), as is common with other monoaminergic neurotransmitters and neuromodulators of the central nervous system. The fact that histamine requires such a conversion prior to oxidative deamination is intriguing since MAO-B is known to be relatively promiscuous towards monoaminergic substrates; its in-vitro oxidation of N-methylhistamine is about 10 times faster than that for histamine, yet this rather subtle difference appears to be governing the decomposition pathway. This work clarifies the MAO-B selectivity toward histamine and N-methylhistamine by multiscale simulations of the rate-limiting hydride abstraction step for both compounds in the gas phase, in aqueous solution, and in the enzyme, using the established empirical valence bond methodology, assisted by gas-phase density functional theory (DFT) calculations. The computed barriers are in very good agreement with experimental kinetic data, especially for relative trends among systems, thereby reproducing the observed MAO-B selectivity. Simulations clearly demonstrate that solvation effects govern the reactivity, both in aqueous solution as well as in the enzyme although with an opposing effect on the free energy barrier. In the aqueous solution, the transition-state structure involving histamine is better solvated than its methylated analog, leading to a lower barrier for histamine oxidation. In the enzyme, the higher hydrophobicity of N-methylhistamine results in a decreased number of water molecules at the active side, leading to decreased dielectric shielding of the preorganized catalytic electrostatic environment provided by the enzyme. This renders the catalytic environment more efficient for N-methylhistamine, giving rise to a lower barrier relative to histamine. In addition, the transition state involving N-methylhistamine appears to be stabilized by the surrounding nonpolar residues to a larger extent than with unsubstituted histamine, contributing to a lower barrier with the former.
Keywords: DFT calculations; N-methylhistamine; QM/MM; activation free energy; empirical valence bond; histamine; metabolic pathway; monoamine oxidase B; multiscale molecular simulations; rate constant; selectivity.
Conflict of interest statement
The authors declare no conflict of interest..
Figures





Similar articles
-
What a Difference a Methyl Group Makes: The Selectivity of Monoamine Oxidase B Towards Histamine and N-Methylhistamine.Chemistry. 2017 Feb 24;23(12):2915-2925. doi: 10.1002/chem.201605430. Epub 2017 Jan 31. Chemistry. 2017. PMID: 28052533
-
Tele-Methylhistamine is a specific MAO B substrate in man.Psychopharmacology (Berl). 1980;69(3):287-90. doi: 10.1007/BF00433097. Psychopharmacology (Berl). 1980. PMID: 6774369
-
Monoamine oxidase-dependent histamine catabolism accounts for post-ischemic cardiac redox imbalance and injury.Biochim Biophys Acta Mol Basis Dis. 2018 Sep;1864(9 Pt B):3050-3059. doi: 10.1016/j.bbadis.2018.06.018. Epub 2018 Jun 25. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29953926
-
The Use of Multiscale Molecular Simulations in Understanding a Relationship between the Structure and Function of Biological Systems of the Brain: The Application to Monoamine Oxidase Enzymes.Front Neurosci. 2016 Jul 15;10:327. doi: 10.3389/fnins.2016.00327. eCollection 2016. Front Neurosci. 2016. PMID: 27471444 Free PMC article. Review.
-
Aminium cation radical mechanism proposed for monoamine oxidase B catalysis: are there alternatives?Xenobiotica. 1995 Jul;25(7):735-53. doi: 10.3109/00498259509061889. Xenobiotica. 1995. PMID: 7483670 Review.
Cited by
-
Fermented Soybean Paste Attenuates Biogenic Amine-Induced Liver Damage in Obese Mice.Cells. 2023 Mar 6;12(5):822. doi: 10.3390/cells12050822. Cells. 2023. PMID: 36899958 Free PMC article.
-
Brunner syndrome caused by point mutation explained by multiscale simulation of enzyme reaction.Sci Rep. 2022 Dec 19;12(1):21889. doi: 10.1038/s41598-022-26296-7. Sci Rep. 2022. PMID: 36536002 Free PMC article.
-
Histaminergic System Activity in the Central Nervous System: The Role in Neurodevelopmental and Neurodegenerative Disorders.Int J Mol Sci. 2024 Sep 12;25(18):9859. doi: 10.3390/ijms25189859. Int J Mol Sci. 2024. PMID: 39337347 Free PMC article. Review.
-
Rumors of Psychedelics, Psychotropics and Related Derivatives in Vachellia and Senegalia in Contrast with Verified Records in Australian Acacia.Plants (Basel). 2022 Dec 2;11(23):3356. doi: 10.3390/plants11233356. Plants (Basel). 2022. PMID: 36501395 Free PMC article. Review.
-
Deciphering the Two-Step Hydride Mechanism of Monoamine Oxidase Flavoenzymes.ACS Omega. 2024 Oct 10;9(42):43046-43057. doi: 10.1021/acsomega.4c06575. eCollection 2024 Oct 22. ACS Omega. 2024. PMID: 39464429 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

