Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity
- PMID: 35163838
- PMCID: PMC8837080
- DOI: 10.3390/ijms23031912
Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity
Abstract
Anthracyclines, such as doxorubicin, are effective chemotherapeutic agents for the treatment of cancer, but their clinical use is associated with severe and potentially life-threatening cardiotoxicity. Despite decades of research, treatment options remain limited. The mitochondria is commonly considered to be the main target of doxorubicin and mitochondrial dysfunction is the hallmark of doxorubicin-induced cardiotoxicity. Here, we review the pathogenic mechanisms of doxorubicin-induced cardiotoxicity and present an update on cardioprotective strategies for this disorder. Specifically, we focus on strategies that can protect the mitochondria and cover different therapeutic modalities encompassing small molecules, post-transcriptional regulators, and mitochondrial transfer. We also discuss the shortcomings of existing models of doxorubicin-induced cardiotoxicity and explore advances in the use of human pluripotent stem cell derived cardiomyocytes as a platform to facilitate the identification of novel treatments against this disorder.
Keywords: anthracyclines; cardiotoxicity; doxorubicin (DOX); hPSC-cardiomyocytes; human pluripotent stem cells (hPSC); mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
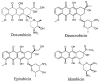
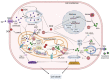
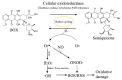
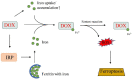

References
-
- Lipshultz S.E., Rifai N., Dalton V.M., Levy D.E., Silverman L.B., Lipsitz S.R., Colan S.D., Asselin B.L., Barr R.D., Clavell L.A., et al. The Effect of Dexrazoxane on Myocardial Injury in Doxorubicin-Treated Children with Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. - DOI - PubMed
-
- Kolaric K., Bradamante V., Cervek J., Cieslinska A., Cisarz-Filipcak E., Denisov L.E., Donat D., Drosik K., Gershanovic M., Hudziec P., et al. A Phase II Trial of Cardioprotection with Cardioxane (ICRF-187) in Patients with Advanced Breast Cancer Receiving 5-Fluorouracil, Doxorubicin and Cyclophosphamide. Oncology. 1995;52:251–255. doi: 10.1159/000227467. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

