Antimicrobial Activity and DFT Studies of a Novel Set of Spiropyrrolidines Tethered with Thiochroman-4-one/Chroman-4-one Scaffolds
- PMID: 35163847
- PMCID: PMC8839074
- DOI: 10.3390/molecules27030582
Antimicrobial Activity and DFT Studies of a Novel Set of Spiropyrrolidines Tethered with Thiochroman-4-one/Chroman-4-one Scaffolds
Abstract
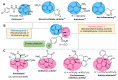
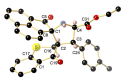
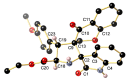
A novel series of 14 spiropyrrolidines bearing thiochroman-4-one/chroman-4-one, and oxindole/acenaphthylene-1,2-dione moieties were synthesized and characterized by spectroscopic techniques, as well as by three X-ray diffraction studies, corroborating the stereochemistry. Quantum chemical calculations studies, using the DFT approach, were performed to rationalize the stereochemical outcome. These N-heterocycles were evaluated for their antibacterial and antifungal activities against some pathogenic organisms. Several compounds displayed moderate to excellent activity towards the screened microbe strains in the study compared to Amoxicillin (AMX), Ampicillin (AMP), and Amphotericin B. Furthermore, a structural activity relationship (SAR) was established considering the synthesized compounds. Pharmacokinetic studies reveal that these derivatives exhibit an acceptable predictive ADMET profile (Absorption, Distribution, Metabolism, Excretion and Toxicity) and good drug-likeness.
Keywords: DFT; [3+2] cycloaddition; chroman-4-one; crystal structure; spiropyrrolidine; thiochroman-4-one.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- WHO Antimicrobial Resistance. 2018. [(accessed on 30 April 2014)]. Available online: www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
-
- Toumi A., Boudriga S., Hamden K., Sobeh M., Cheurfa M., Askri M., Knorr M., Strohmann B.C.L. Synthesis, antidiabetic activity and molecular docking study of rhodanine-substitued spirooxindole pyrrolidine derivatives as novel α-amylase inhibitors. Bioorg. Chem. 2021;106:104507. doi: 10.1016/j.bioorg.2020.104507. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

