Prion Strains Differ in Susceptibility to Photodynamic Oxidation
- PMID: 35163872
- PMCID: PMC8840242
- DOI: 10.3390/molecules27030611
Prion Strains Differ in Susceptibility to Photodynamic Oxidation
Abstract
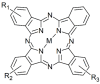
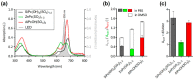
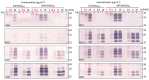
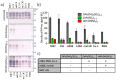
Prion disorders, or transmissible spongiform encephalophaties (TSE), are fatal neurodegenerative diseases affecting mammals. Prion-infectious particles comprise of misfolded pathological prion proteins (PrPTSE). Different TSEs are associated with distinct PrPTSE folds called prion strains. The high resistance of prions to conventional sterilization increases the risk of prion transmission in medical, veterinary and food industry practices. Recently, we have demonstrated the ability of disulfonated hydroxyaluminum phthalocyanine to photodynamically inactivate mouse RML prions by generated singlet oxygen. Herein, we studied the efficiency of three phthalocyanine derivatives in photodynamic treatment of seven mouse adapted prion strains originating from sheep, human, and cow species. We report the different susceptibilities of the strains to photodynamic oxidative elimination of PrPTSE epitopes: RML, A139, Fu-1 > mBSE, mvCJD > ME7, 22L. The efficiency of the phthalocyanine derivatives in the epitope elimination also differed (AlPcOH(SO3)2 > ZnPc(SO3)1-3 > SiPc(OH)2(SO3)1-3) and was not correlated to the yields of generated singlet oxygen. Our data suggest that the structural properties of both the phthalocyanine and the PrPTSE strain may affect the effectiveness of the photodynamic prion inactivation. Our finding provides a new option for the discrimination of prion strains and highlights the necessity of utilizing range of prion strains when validating the photodynamic prion decontamination procedures.
Keywords: PDI; PrP; TSE; photodynamic; phthalocyanine; prion; prion inactivation; protein folding; singlet oxygen; strain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Optimization of the photodynamic inactivation of prions by a phthalocyanine photosensitizer: The crucial involvement of singlet oxygen.J Biophotonics. 2019 Aug;12(8):e201800340. doi: 10.1002/jbio.201800430. Epub 2019 May 2. J Biophotonics. 2019. PMID: 30989822
-
In Vitro Approach To Identify Key Amino Acids in Low Susceptibility of Rabbit Prion Protein to Misfolding.J Virol. 2017 Nov 30;91(24):e01543-17. doi: 10.1128/JVI.01543-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978705 Free PMC article.
-
Neuropathology of Animal Prion Diseases.Biomolecules. 2021 Mar 21;11(3):466. doi: 10.3390/biom11030466. Biomolecules. 2021. PMID: 33801117 Free PMC article. Review.
-
Ovine recombinant PrP as an inhibitor of ruminant prion propagation in vitro.Prion. 2017 Jul 4;11(4):265-276. doi: 10.1080/19336896.2017.1342919. Epub 2017 Jun 30. Prion. 2017. PMID: 28665745 Free PMC article.
-
The cellular and pathologic prion protein.Handb Clin Neurol. 2018;153:21-44. doi: 10.1016/B978-0-444-63945-5.00002-7. Handb Clin Neurol. 2018. PMID: 29887138 Review.
Cited by
-
Expression of Toll-like receptors in the cerebellum during pathogenesis of prion disease.Front Behav Neurosci. 2024 Apr 18;18:1341901. doi: 10.3389/fnbeh.2024.1341901. eCollection 2024. Front Behav Neurosci. 2024. PMID: 38698886 Free PMC article.
References
-
- Lehmann S., Pastore M., Rogez-Kreuz C., Richard M., Belondrade M., Rauwel G., Durand F., Yousfi R., Criquelion J., Clayette P., et al. New hospital disinfection processes for both conventional and prion infectious agents compatible with thermosensitive medical equipment. J. Hosp. Infect. 2009;72:342–350. doi: 10.1016/j.jhin.2009.03.024. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

