Design, Synthesis, Spectroscopic Characterisation and In Vitro Cytostatic Evaluation of Novel Bis(coumarin-1,2,3-triazolyl)benzenes and Hybrid Coumarin-1,2,3-triazolyl-aryl Derivatives
- PMID: 35163905
- PMCID: PMC8840664
- DOI: 10.3390/molecules27030637
Design, Synthesis, Spectroscopic Characterisation and In Vitro Cytostatic Evaluation of Novel Bis(coumarin-1,2,3-triazolyl)benzenes and Hybrid Coumarin-1,2,3-triazolyl-aryl Derivatives
Abstract
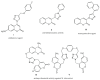
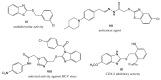
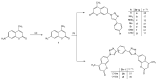
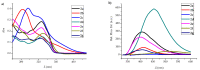
In this work, a series of novel 1,2,3-triazolyl-coumarin hybrid systems were designed as potential antitumour agents. The structural modification of the coumarin ring was carried out by Cu(I)-catalysed Huisgen 1,3-dipolar cycloaddition of 7-azido-4-methylcoumarin and terminal aromatic alkynes to obtain 1,4-disubstituted 1,2,3-triazolyl-coumarin conjugates 2a-g, bis(1,2,3-triazolyl-coumarin)benzenes 2h-i and coumarin-1,2,3-triazolyl-benzazole hybrids 4a-b. The newly synthesised hybrid molecules were investigated for in vitro antitumour activity against five human cancer cell lines, colon carcinoma HCT116, breast carcinoma MCF-7, lung carcinoma H 460, human T-lymphocyte cells CEM, cervix carcinoma cells HeLa, as well as human dermal microvascular endothelial cells (HMEC-1). Most of these compounds showed moderate to pronounced cytotoxic activity, especially towards MCF-7 cell lines with IC50 = 0.3-32 μM. In addition, compounds 2a-i and 4a-b were studied by UV-Vis absorption and fluorescence spectroscopy and their basic photophysical parameters were determined.
Keywords: 1,2,3-triazole; benzazole; coumarin; cytostatic evaluation; fluorescence; organic synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole-1,2,3-triazole-sulfonamide hybrids as antimicrobial and antiproliferative agents.Chem Cent J. 2018 Nov 1;12(1):110. doi: 10.1186/s13065-018-0479-1. Chem Cent J. 2018. PMID: 30387018 Free PMC article.
-
Synthesis and in vitro evaluation of antiviral and cytostatic properties of novel 8-triazolyl acyclovir derivatives.Nucleosides Nucleotides Nucleic Acids. 2018;37(7):397-414. doi: 10.1080/15257770.2018.1485932. Epub 2018 Nov 17. Nucleosides Nucleotides Nucleic Acids. 2018. PMID: 30449256
-
6-(4'-Aryl-1',2',3'-triazolyl)-spirostan-3,5-diols and 6-(4'-Aryl-1',2',3'-triazolyl)-7-hydroxyspirosta-1,4-dien-3-ones: Synthesis and analysis of their cytotoxicity.Steroids. 2019 Nov;151:108460. doi: 10.1016/j.steroids.2019.108460. Epub 2019 Jul 22. Steroids. 2019. PMID: 31344410
-
Thiazolidinone-linked1,2,3-triazoles with monoterpenic skeleton as new potential anticancer agents: Design, synthesis and molecular docking studies.Bioorg Chem. 2021 Oct;115:105184. doi: 10.1016/j.bioorg.2021.105184. Epub 2021 Jul 20. Bioorg Chem. 2021. PMID: 34333421 Review.
-
Coumarin Hybrids: Promising Scaffolds in the Treatment of Breast Cancer.Mini Rev Med Chem. 2019;19(17):1443-1458. doi: 10.2174/1389557519666190308122509. Mini Rev Med Chem. 2019. PMID: 30854961 Review.
Cited by
-
Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure-Activity Relationships.Biomedicines. 2024 May 27;12(6):1192. doi: 10.3390/biomedicines12061192. Biomedicines. 2024. PMID: 38927399 Free PMC article. Review.
-
Solvatomorphic Diversity in Coordination Compounds of Copper(II) with l-Homoserine and 1,10-Phenanthroline: Syntheses, Crystal Structures and ESR Study.Molecules. 2024 Nov 27;29(23):5621. doi: 10.3390/molecules29235621. Molecules. 2024. PMID: 39683780 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

