Approaches to the Potential Therapy of COVID-19: A General Overview from the Medicinal Chemistry Perspective
- PMID: 35163923
- PMCID: PMC8838458
- DOI: 10.3390/molecules27030658
Approaches to the Potential Therapy of COVID-19: A General Overview from the Medicinal Chemistry Perspective
Abstract
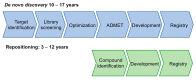
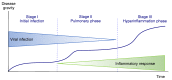
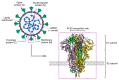
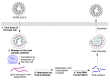
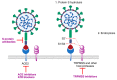
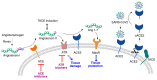
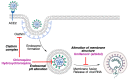
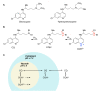
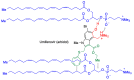
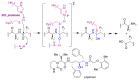
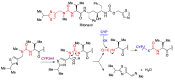
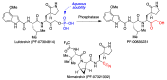
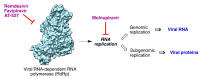
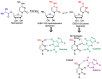
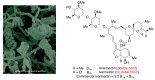
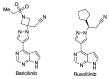
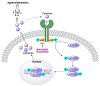
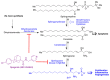
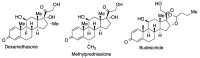
In spite of advances in vaccination, control of the COVID-19 pandemic will require the use of pharmacological treatments against SARS-CoV2. Their development needs to consider the existence of two phases in the disease, namely the viral infection and the inflammatory stages. The main targets for antiviral therapeutic intervention are: (a) viral proteins, including the spike (S) protein characteristic of the viral cover and the viral proteases in charge of processing the polyprotein arising from viral genome translation; (b) host proteins, such as those involved in the processes related to viral entry into the host cell and the release of the viral genome inside the cell, the elongation factor eEF1A and importins. The use of antivirals targeted at host proteins is less developed but it has the potential advantage of not being affected by mutations in the genome of the virus and therefore being active against all its variants. Regarding drugs that address the hyperinflammatory phase of the disease triggered by the so-called cytokine storm, the following strategies are particularly relevant: (a) drugs targeting JAK kinases; (b) sphingosine kinase 2 inhibitors; (c) antibodies against interleukin 6 or its receptor; (d) use of the traditional anti-inflammatory corticosteroids.
Keywords: 3CLpro inhibitors; IL6 antibodies; JAK-STAT inhibitors; RdRp inhibitors; S antibodies; SARS-CoV-2; SphK2 inhibitors; corticoids; serine protease inhibitors.
Conflict of interest statement
The author declares no conflict of interest.
Figures
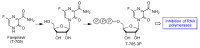
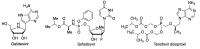

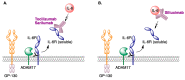
Similar articles
-
TMPRSS2 and RNA-Dependent RNA Polymerase Are Effective Targets of Therapeutic Intervention for Treatment of COVID-19 Caused by SARS-CoV-2 Variants (B.1.1.7 and B.1.351).Microbiol Spectr. 2021 Sep 3;9(1):e0047221. doi: 10.1128/Spectrum.00472-21. Epub 2021 Aug 11. Microbiol Spectr. 2021. PMID: 34378968 Free PMC article.
-
Potential inhibitors of SARS-CoV-2: recent advances.J Drug Target. 2021 Apr;29(4):349-364. doi: 10.1080/1061186X.2020.1853736. Epub 2020 Dec 3. J Drug Target. 2021. PMID: 33210953 Review.
-
Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: A systematic review of in vitro and in vivo studies.J Cell Physiol. 2021 Apr;236(4):2364-2392. doi: 10.1002/jcp.30032. Epub 2020 Sep 9. J Cell Physiol. 2021. PMID: 32901936
-
Overview of antiviral drug candidates targeting coronaviral 3C-like main proteases.FEBS J. 2021 Sep;288(17):5089-5121. doi: 10.1111/febs.15696. Epub 2021 Feb 1. FEBS J. 2021. PMID: 33400393 Review.
-
Cancer vs. SARS-CoV-2 induced inflammation, overlapping functions, and pharmacological targeting.Inflammopharmacology. 2021 Apr;29(2):343-366. doi: 10.1007/s10787-021-00796-w. Epub 2021 Mar 15. Inflammopharmacology. 2021. PMID: 33723711 Free PMC article. Review.
Cited by
-
Cyanobacteria and Algae-Derived Bioactive Metabolites as Antiviral Agents: Evidence, Mode of Action, and Scope for Further Expansion; A Comprehensive Review in Light of the SARS-CoV-2 Outbreak.Antioxidants (Basel). 2022 Feb 10;11(2):354. doi: 10.3390/antiox11020354. Antioxidants (Basel). 2022. PMID: 35204236 Free PMC article. Review.
-
The effect of various compounds on the COVID mechanisms, from chemical to molecular aspects.Biophys Chem. 2022 Sep;288:106824. doi: 10.1016/j.bpc.2022.106824. Epub 2022 May 12. Biophys Chem. 2022. PMID: 35728510 Free PMC article. Review.
-
Computational discovery of dual potential inhibitors of SARS-CoV-2 spike/ACE2 and Mpro: 3D-pharmacophore, docking-based virtual screening, quantum mechanics and molecular dynamics.Eur Biophys J. 2024 Aug;53(5-6):277-298. doi: 10.1007/s00249-024-01713-z. Epub 2024 Jun 21. Eur Biophys J. 2024. PMID: 38907013
-
Roles of host proteases in the entry of SARS-CoV-2.Anim Dis. 2023;3(1):12. doi: 10.1186/s44149-023-00075-x. Epub 2023 Apr 25. Anim Dis. 2023. PMID: 37128508 Free PMC article. Review.
-
Therapeutic Polypeptides and Peptidomimetics: Powerful Tools for COVID-19 Treatment.Clin Drug Investig. 2023 Jan;43(1):13-22. doi: 10.1007/s40261-022-01231-w. Epub 2022 Dec 3. Clin Drug Investig. 2023. PMID: 36462104 Free PMC article. Review.
References
-
- COVID-19 Vaccine and Therapeutics Tracker. [(accessed on 31 December 2021)]. Available online: https://biorender.com/covid-vaccine-tracker/details/d-0118/interferon-alpha.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

