Structural Refinement of 2,4-Thiazolidinedione Derivatives as New Anticancer Agents Able to Modulate the BAG3 Protein
- PMID: 35163936
- PMCID: PMC8839660
- DOI: 10.3390/molecules27030665
Structural Refinement of 2,4-Thiazolidinedione Derivatives as New Anticancer Agents Able to Modulate the BAG3 Protein
Abstract
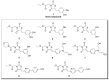
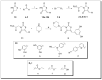
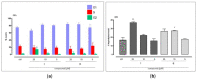
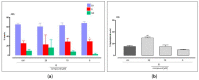
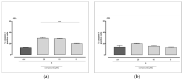
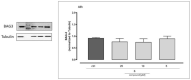
The multidomain BAG3 protein is a member of the BAG (Bcl-2-associated athanogene) family of co-chaperones, involved in a wide range of protein-protein interactions crucial for many key cellular pathways, including autophagy, cytoskeletal dynamics, and apoptosis. Basal expression of BAG3 is elevated in several tumor cell lines, where it promotes cell survival signaling and apoptosis resistance through the interaction with many protein partners. In addition, its role as a key player of several hallmarks of cancer, such as metastasis, angiogenesis, autophagy activation, and apoptosis inhibition, has been established. Due to its involvement in malignant transformation, BAG3 has emerged as a potential and effective biological target to control multiple cancer-related signaling pathways. Recently, by using a multidisciplinary approach we reported the first synthetic BAG3 modulator interfering with its BAG domain (BD), based on a 2,4-thiazolidinedione scaffold and endowed with significant anti-proliferative activity. Here, a further in silico-driven selection of a 2,4-thiazolidinedione-based compound was performed. Thanks to a straightforward synthesis, relevant binding affinity for the BAG3BD domain, and attractive biological activities, this novel generation of compounds is of great interest for the development of further BAG3 binders, as well as for the elucidation of the biological roles of this protein in tumors. Specifically, we found compound 6 as a new BAG3 modulator with a relevant antiproliferative effect on two different cancer cell lines (IC50: A375 = 19.36 μM; HeLa = 18.67 μM).
Keywords: 2,4-thiazolidinedione scaffold; BAG domain modulators; BAG3 protein; cancer; chaperones; surface plasmon resonance (SPR) assay; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Identification of a New Promising BAG3 Modulator Featuring the Imidazopyridine Scaffold.Molecules. 2024 Oct 25;29(21):5051. doi: 10.3390/molecules29215051. Molecules. 2024. PMID: 39519692 Free PMC article.
-
Design, synthesis and biological evaluation of novel 2,4-thiazolidinedione derivatives able to target the human BAG3 protein.Eur J Med Chem. 2023 Dec 5;261:115824. doi: 10.1016/j.ejmech.2023.115824. Epub 2023 Sep 22. Eur J Med Chem. 2023. PMID: 37783101
-
Discovery and synthesis of the first selective BAG domain modulator of BAG3 as an attractive candidate for the development of a new class of chemotherapeutics.Chem Commun (Camb). 2018 Jul 5;54(55):7613-7616. doi: 10.1039/c8cc03399d. Chem Commun (Camb). 2018. PMID: 29926854
-
BAG3: a multifaceted protein that regulates major cell pathways.Cell Death Dis. 2011 Apr 7;2(4):e141. doi: 10.1038/cddis.2011.24. Cell Death Dis. 2011. PMID: 21472004 Free PMC article. Review.
-
At the Crossroads of Apoptosis and Autophagy: Multiple Roles of the Co-Chaperone BAG3 in Stress and Therapy Resistance of Cancer.Cells. 2020 Feb 28;9(3):574. doi: 10.3390/cells9030574. Cells. 2020. PMID: 32121220 Free PMC article. Review.
Cited by
-
Identification of a New Promising BAG3 Modulator Featuring the Imidazopyridine Scaffold.Molecules. 2024 Oct 25;29(21):5051. doi: 10.3390/molecules29215051. Molecules. 2024. PMID: 39519692 Free PMC article.
-
Chemoproteomics Reveals USP5 (Ubiquitin Carboxyl-Terminal Hydrolase 5) as Promising Target of the Marine Polyketide Gracilioether A.Mar Drugs. 2024 Jan 11;22(1):41. doi: 10.3390/md22010041. Mar Drugs. 2024. PMID: 38248666 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

