Synthesis, Antimicrobial, Anti-Virulence and Anticancer Evaluation of New 5(4 H)-Oxazolone-Based Sulfonamides
- PMID: 35163939
- PMCID: PMC8838850
- DOI: 10.3390/molecules27030671
Synthesis, Antimicrobial, Anti-Virulence and Anticancer Evaluation of New 5(4 H)-Oxazolone-Based Sulfonamides
Abstract
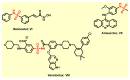
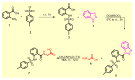
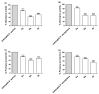
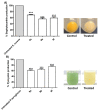
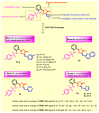
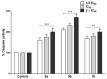
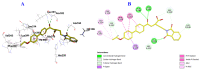
Since the synthesis of prontosil the first prodrug shares their chemical moiety, sulfonamides exhibit diverse modes of actions to serve as antimicrobials, diuretics, antidiabetics, and other clinical applications. This inspiring chemical nucleus has promoted several research groups to investigate the synthesis of new members exploring new clinical applications. In this study, a novel series of 5(4H)-oxazolone-based-sulfonamides (OBS) 9a-k were synthesized, and their antibacterial and antifungal activities were evaluated against a wide range of Gram-positive and -negative bacteria and fungi. Most of the tested compounds exhibited promising antibacterial activity against both Gram-positive and -negative bacteria particularly OBS 9b and 9f. Meanwhile, compound 9h showed the most potent antifungal activity. Moreover, the OBS 9a, 9b, and 9f that inhibited the bacterial growth at the lowest concentrations were subjected to further evaluation for their anti-virulence activities against Pseudomonas aeruginosa and Staphylococcus aureus. Interestingly, the three tested compounds reduced the biofilm formation and diminished the production of virulence factors in both P. aeruginosa and S. aureus. Bacteria use a signaling system, quorum sensing (QS), to regulate their virulence. In this context, in silico study has been conducted to assess the ability of OBS to compete with the QS receptors. The tested OBS showed marked ability to bind and hinder QS receptors, indicating that anti-virulence activities of OBS could be due to blocking QS, the system that controls the bacterial virulence. Furthermore, anticancer activity has been further performed for such derivatives. The OBS compounds showed variable anti-tumor activities, specifically 9a, 9b, 9f and 9k, against different cancer lines. Conclusively, the OBS compounds can serve as antimicrobials, anti-virulence and anti-tumor agents.
Keywords: anti-virulence; antibiofilm; anticancer; antimicrobial; oxazolone; sulfonamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Antipathogenic Compounds That Are Effective at Very Low Concentrations and Have Both Antibiofilm and Antivirulence Effects against Pseudomonas aeruginosa.Microbiol Spectr. 2021 Oct 31;9(2):e0024921. doi: 10.1128/Spectrum.00249-21. Epub 2021 Sep 8. Microbiol Spectr. 2021. PMID: 34494853 Free PMC article.
-
Antimicrobial and anti-virulence activities of 4-shogaol from grains of paradise against gram-negative bacteria: Integration of experimental and computational methods.J Ethnopharmacol. 2024 Apr 6;323:117611. doi: 10.1016/j.jep.2023.117611. Epub 2023 Dec 27. J Ethnopharmacol. 2024. PMID: 38158095
-
Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model.PLoS One. 2017 Apr 28;12(4):e0176883. doi: 10.1371/journal.pone.0176883. eCollection 2017. PLoS One. 2017. PMID: 28453568 Free PMC article.
-
Eugenol as a promising antibiofilm and anti-quorum sensing agent: A systematic review.Microb Pathog. 2024 Nov;196:106937. doi: 10.1016/j.micpath.2024.106937. Epub 2024 Sep 16. Microb Pathog. 2024. PMID: 39293727
-
Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors.Microb Pathog. 2021 Jun;155:104912. doi: 10.1016/j.micpath.2021.104912. Epub 2021 Apr 28. Microb Pathog. 2021. PMID: 33932548 Review.
Cited by
-
Biofilm Lifestyle in Recurrent Urinary Tract Infections.Life (Basel). 2023 Jan 4;13(1):148. doi: 10.3390/life13010148. Life (Basel). 2023. PMID: 36676100 Free PMC article. Review.
-
Sodium Citrate Alleviates Virulence in Pseudomonas aeruginosa.Microorganisms. 2022 May 18;10(5):1046. doi: 10.3390/microorganisms10051046. Microorganisms. 2022. PMID: 35630488 Free PMC article.
-
Analyzing the metabolic fate of oral administration drugs: A review and state-of-the-art roadmap.Front Pharmacol. 2022 Oct 7;13:962718. doi: 10.3389/fphar.2022.962718. eCollection 2022. Front Pharmacol. 2022. PMID: 36278150 Free PMC article. Review.
-
Diminishing the Pathogenesis of the Food-Borne Pathogen Serratia marcescens by Low Doses of Sodium Citrate.Biology (Basel). 2023 Mar 26;12(4):504. doi: 10.3390/biology12040504. Biology (Basel). 2023. PMID: 37106705 Free PMC article.
-
Quercetin: a promising virulence inhibitor of Pseudomonas aeruginosa LasB in vitro.Appl Microbiol Biotechnol. 2024 Dec;108(1):57. doi: 10.1007/s00253-023-12890-w. Epub 2024 Jan 5. Appl Microbiol Biotechnol. 2024. PMID: 38180553 Free PMC article.
References
-
- Ibrahim T.S., Almalki A.J., Moustafa A.H., Allam R.M., Abuo-Rahma G.E.-D.A., El Subbagh H.I., Mohamed M.F.A. Novel 1,2,4-oxadiazole-chalcone/oxime hybrids as potential antibacterial DNA gyrase inhibitors: Design, synthesis, ADMET prediction and molecular docking study. Bioorg. Chem. 2021;111:104885. doi: 10.1016/j.bioorg.2021.104885. - DOI - PubMed
-
- Hofny H.A., Mohamed M.F.A., Gomaa H.A.M., Abdel-Aziz S.A., Youssif B.G.M., El-koussi N.A., Aboraia A.S. Design, synthesis, and antibacterial evaluation of new quinoline-1,3,4-oxadiazole and quinoline-1,2,4-triazole hybrids as potential inhibitors of DNA gyrase and topoisomerase IV. Bioorg. Chem. 2021;112:104920. doi: 10.1016/j.bioorg.2021.104920. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

