Enantiotropy of Simvastatin as a Result of Weakened Interactions in the Crystal Lattice: Entropy-Driven Double Transitions and the Transient Modulated Phase as Seen by Solid-State NMR Spectroscopy
- PMID: 35163943
- PMCID: PMC8838109
- DOI: 10.3390/molecules27030679
Enantiotropy of Simvastatin as a Result of Weakened Interactions in the Crystal Lattice: Entropy-Driven Double Transitions and the Transient Modulated Phase as Seen by Solid-State NMR Spectroscopy
Abstract
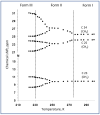
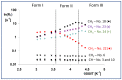
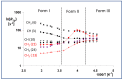
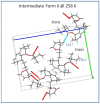
In crystalline molecular solids, in the absence of strong intermolecular interactions, entropy-driven processes play a key role in the formation of dynamically modulated transient phases. Specifically, in crystalline simvastatin, the observed fully reversible enantiotropic behavior is associated with multiple order-disorder transitions: upon cooling, the dynamically disordered high-temperature polymorphic Form I is transformed to the completely ordered low-temperature polymorphic Form III via the intermediate (transient) modulated phase II. This behavior is associated with a significant reduction in the kinetic energy of the rotating and flipping ester substituents, as well as a decrease in structural ordering into two distinct positions. In transient phase II, the conventional three-dimensional structure is modulated by periodic distortions caused by cooperative conformation exchange of the ester substituent between the two states, which is enabled by weakened hydrogen bonding. Based on solid-state NMR data analysis, the mechanism of the enantiotropic phase transition and the presence of the transient modulated phase are documented.
Keywords: dynamics; enantiotropy; entropy; polymorphism; solid-state nuclear magnetic resonance (NMR); transient modulated phase.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures








Similar articles
-
Polymorphism in Simvastatin: Twinning, Disorder, and Enantiotropic Phase Transitions.Mol Pharm. 2018 Nov 5;15(11):5349-5360. doi: 10.1021/acs.molpharmaceut.8b00818. Epub 2018 Oct 4. Mol Pharm. 2018. PMID: 30230340
-
Probing phase transitions in simvastatin with terahertz time-domain spectroscopy.Mol Pharm. 2015 Mar 2;12(3):810-5. doi: 10.1021/mp500649q. Epub 2015 Feb 3. Mol Pharm. 2015. PMID: 25615410
-
Phase transitions in the crystals of L- and DL-cysteine on cooling: intermolecular hydrogen bonds distortions and the side-chain motions of thiol-groups. 1. L-cysteine.J Phys Chem B. 2008 Oct 9;112(40):12827-39. doi: 10.1021/jp804142c. Epub 2008 Sep 13. J Phys Chem B. 2008. PMID: 18793012
-
General principles of pharmaceutical solid polymorphism: a supramolecular perspective.Adv Drug Deliv Rev. 2004 Feb 23;56(3):241-74. doi: 10.1016/j.addr.2003.10.005. Adv Drug Deliv Rev. 2004. PMID: 14962581 Review.
-
A review of nanocrystalline cellulose suspensions: Rheology, liquid crystal ordering and colloidal phase behaviour.Adv Colloid Interface Sci. 2020 Jan;275:102076. doi: 10.1016/j.cis.2019.102076. Epub 2019 Nov 19. Adv Colloid Interface Sci. 2020. PMID: 31780045 Review.
Cited by
-
Spiers Memorial Lecture: NMR crystallography.Faraday Discuss. 2025 Jan 8;255(0):9-45. doi: 10.1039/d4fd00151f. Faraday Discuss. 2025. PMID: 39405130 Free PMC article.
-
An advanced approach combining solid-state NMR with powder diffraction applied to newly synthesized iso-thio-uronium salts.J Appl Crystallogr. 2025 Feb 11;58(Pt 2):321-332. doi: 10.1107/S1600576724012378. eCollection 2025 Apr 1. J Appl Crystallogr. 2025. PMID: 40170969 Free PMC article.
References
-
- Griesser U.J., Jetti R.K.R., Haddow M.F., Brehmer T., Apperley D.C., King A., Harris R.K. Conformational Polymorphism in Oxybuprocaine Hydrochloride. Cryst. Growth Des. 2008;8:44–56. doi: 10.1021/cg070590d. - DOI
-
- Falls Z., Avery P., Wang X., Hilleke K.P., Zurek E. The XtalOpt Evolutionary Algorithm for Crystal Structure Prediction. J. Phys. Chem. C. 2021;125:1601–1620. doi: 10.1021/acs.jpcc.0c09531. - DOI
-
- Hušák M., Kratochvíl B., Jegorov A., Brus J., Maixner J., Rohlicek J. Simvastatin: Structure solution of two new low-temperature phases from synchrotron powder diffraction and ss-NMR. Struct. Chem. 2010;21:511–518. doi: 10.1007/s11224-009-9579-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

