Polyextremophilic Chitinolytic Activity by a Marine Strain (IG119) of Clonostachys rosea
- PMID: 35163952
- PMCID: PMC8838608
- DOI: 10.3390/molecules27030688
Polyextremophilic Chitinolytic Activity by a Marine Strain (IG119) of Clonostachys rosea
Abstract
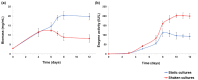
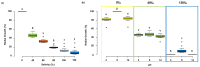
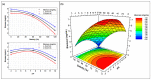
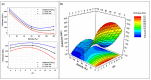
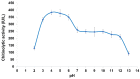
The investigation for novel unique extremozymes is a valuable business for which the marine environment has been overlooked. The marine fungus Clonostachys rosea IG119 was tested for growth and chitinolytic enzyme production at different combinations of salinity and pH using response surface methodology. RSM modelling predicted best growth in-between pH 3.0 and 9.0 and at salinity of 0-40‱, and maximum enzyme activity (411.137 IU/L) at pH 6.4 and salinity 0‱; however, quite high production (>390 IU/L) was still predicted at pH 4.5-8.5. The highest growth and activity were obtained, respectively, at pH 4.0 and 8.0, in absence of salt. The crude enzyme was tested at different salinities (0-120‱) and pHs (2.0-13.0). The best activity was achieved at pH 4.0, but it was still high (in-between 3.0 and 12.0) at pH 2.0 and 13.0. Salinity did not affect the activity in all tested conditions. Overall, C. rosea IG119 was able to grow and produce chitinolytic enzymes under polyextremophilic conditions, and its crude enzyme solution showed more evident polyextremophilic features. The promising chitinolytic activity of IG119 and the peculiar characteristics of its chitinolytic enzymes could be suitable for several biotechnological applications (i.e., degradation of salty chitin-rich materials and biocontrol of spoiling organisms, possibly solving some relevant environmental issues).
Keywords: Clonostachys rosea; chitinolytic enzymes; high producer; marine fungi; polyextremophiles; response surface methodology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
High Production of Chitinolytic Activity in Halophilic Conditions by a New Marine Strain of Clonostachys rosea.Molecules. 2019 May 16;24(10):1880. doi: 10.3390/molecules24101880. Molecules. 2019. PMID: 31100818 Free PMC article.
-
The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes.Molecules. 2016 Apr 5;21(4):447. doi: 10.3390/molecules21040447. Molecules. 2016. PMID: 27058517 Free PMC article. Review.
-
Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003.Microb Cell Fact. 2012 Jan 23;11:12. doi: 10.1186/1475-2859-11-12. Microb Cell Fact. 2012. PMID: 22270226 Free PMC article.
-
Marine chitinolytic enzymes, a biotechnological treasure hidden in the ocean?Appl Microbiol Biotechnol. 2018 Dec;102(23):9937-9948. doi: 10.1007/s00253-018-9385-7. Epub 2018 Oct 1. Appl Microbiol Biotechnol. 2018. PMID: 30276711 Review.
-
Effects of culture conditions on spore types of Clonostachys rosea 67-1 in submerged fermentation.Lett Appl Microbiol. 2014 Apr;58(4):318-24. doi: 10.1111/lam.12193. Epub 2013 Nov 29. Lett Appl Microbiol. 2014. PMID: 24286624
Cited by
-
From marine neglected substrata new fungal taxa of potential biotechnological interest: the case of Pelagia noctiluca.Front Microbiol. 2024 Oct 11;15:1473269. doi: 10.3389/fmicb.2024.1473269. eCollection 2024. Front Microbiol. 2024. PMID: 39464400 Free PMC article.
-
Rambellisea gigliensis and Rambellisea halocynthiae, gen. et spp. nov. (Lulworthiaceae) from the Marine Tunicate Halocynthia papillosa.J Fungi (Basel). 2024 Feb 3;10(2):127. doi: 10.3390/jof10020127. J Fungi (Basel). 2024. PMID: 38392799 Free PMC article.
-
Transcriptomic Response of Clonostachys rosea Mycoparasitizing Rhizoctonia solani.J Fungi (Basel). 2023 Aug 2;9(8):818. doi: 10.3390/jof9080818. J Fungi (Basel). 2023. PMID: 37623589 Free PMC article.
-
An overview of fungal chitinases and their potential applications.Protoplasma. 2023 Jul;260(4):1031-1046. doi: 10.1007/s00709-023-01839-5. Epub 2023 Feb 8. Protoplasma. 2023. PMID: 36752884 Review.
-
Chitinase-functionalized UiO-66 framework nanoparticles active against multidrug-resistant Candida Auris.BMC Microbiol. 2024 Jul 20;24(1):269. doi: 10.1186/s12866-024-03414-1. BMC Microbiol. 2024. PMID: 39030474 Free PMC article.
References
-
- Barghini P., Pasqualetti M., Gorrasi S., Fenice M. Bacteria from the “Saline di Tarquinia” marine salterns reveal very atypical growth profiles with regards to salinity and temperature. Mediterr. Mar. Sci. 2018;19:513–525. doi: 10.12681/mms.15514. - DOI
-
- Gorrasi S., Pesciaroli C., Barghini P., Pasqualetti M., Fenice M. Structure and diversity of the bacterial community of an Arctic estuarine system (Kandalaksha Bay) subject to intense tidal currents. J. Mar. Syst. 2019;196:77–85. doi: 10.1016/j.jmarsys.2019.04.004. - DOI
-
- Gorrasi S., Franzetti A., Ambrosini R., Pittino F., Pasqualetti M., Fenice M. Spatio-temporal variation of the bacterial communities along a salinity gradient within a thalassohaline environment (Saline di Tarquinia Salterns, Italy) Molecules. 2021;26:1338. doi: 10.3390/molecules26051338. - DOI - PMC - PubMed
-
- Fenice M., Gallo A.M., Juarez-Jimenez B., Gonzalez-Lopez J. Screening for extracellular enzyme activities by bacteria isolated from samples collected in the Tyrrhenian Sea. Ann. Microbiol. 2007;57:93–99. doi: 10.1007/BF03175056. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

