In Vitro and In Silico Study of Analogs of Plant Product Plastoquinone to Be Effective in Colorectal Cancer Treatment
- PMID: 35163957
- PMCID: PMC8839215
- DOI: 10.3390/molecules27030693
In Vitro and In Silico Study of Analogs of Plant Product Plastoquinone to Be Effective in Colorectal Cancer Treatment
Abstract
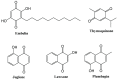
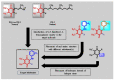
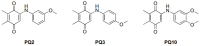
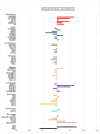
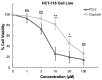
Plants have paved the way for the attainment of molecules with a wide-range of biological activities. However, plant products occasionally show low biological activities and/or poor pharmacokinetic properties. In that case, development of their derivatives as drugs from the plant world has been actively performed. As plant products, plastoquinones (PQs) have been of high importance in anticancer drug design and discovery; we have previously evaluated and reported the potential cytotoxic effects of a series of PQ analogs. Among these analogs, PQ2, PQ3 and PQ10 were selected for National Cancer Institute (NCI) for in vitro screening of anticancer activity against a wide range of cancer cell lines. The apparent superior anticancer potency of PQ2 on the HCT-116 colorectal cancer cell line than that of PQ3 and PQ10 compared to other tested cell lines has encouraged us to perform further mechanistic studies to enlighten the mode of anti-colorectal cancer action of PQ2. For this purpose, its apoptotic effects on the HCT-116 cell line, DNA binding capacity and several crucial pharmacokinetic properties were investigated. Initially, MTT assay was conducted for PQ2 at different concentrations against HCT-116 cells. Results indicated that PQ2 exhibited significant cytotoxicity in HCT-116 cells with an IC50 value of 4.97 ± 1.93 μM compared to cisplatin (IC50 = 26.65 ± 7.85 μM). Moreover, apoptotic effects of PQ2 on HCT-116 cells were investigated by the annexin V/ethidium homodimer III staining method and PQ2 significantly induced apoptosis in HCT-116 cells compared to cisplatin. Based on the potent DNA cleavage capacity of PQ2, molecular docking studies were conducted in the minor groove of the double helix of DNA and PQ2 presented a key hydrogen bonding through its methoxy moiety. Overall, both in vitro and in silico studies indicated that effective, orally bioavailable drug-like PQ2 attracted attention for colorectal cancer treatment. The most important point to emerge from this study is that appropriate derivatization of a plant product leads to unique biologically active compounds.
Keywords: DNA cleavage; apoptosis; colorectal cancer; cytotoxicity; molecular docking; pharmacokinetic properties; plastoquinones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Scaffold Hopping and Structural Modification of NSC 663284: Discovery of Potent (Non)Halogenated Aminobenzoquinones.Biomedicines. 2023 Dec 24;12(1):50. doi: 10.3390/biomedicines12010050. Biomedicines. 2023. PMID: 38255157 Free PMC article.
-
Design, synthesis, and biological activity of Plastoquinone analogs as a new class of anticancer agents.Bioorg Chem. 2019 Nov;92:103255. doi: 10.1016/j.bioorg.2019.103255. Epub 2019 Sep 7. Bioorg Chem. 2019. PMID: 31542717
-
Design, synthesis and investigation of the mechanism of action underlying anti-leukemic effects of the quinolinequinones as LY83583 analogs.Bioorg Chem. 2021 Sep;114:105160. doi: 10.1016/j.bioorg.2021.105160. Epub 2021 Jul 10. Bioorg Chem. 2021. PMID: 34328861
-
2-Anilinopyrimidine derivatives: Design, synthesis, in vitro anti-proliferative activity, EGFR and ARO inhibitory activity, cell cycle analysis and molecular docking study.Bioorg Chem. 2020 Jun;99:103798. doi: 10.1016/j.bioorg.2020.103798. Epub 2020 Mar 29. Bioorg Chem. 2020. PMID: 32247112 Review.
-
Mechanistic Insights into the Anticancer Potential of Methoxyflavones Analogs: A Review.Molecules. 2025 Jan 16;30(2):346. doi: 10.3390/molecules30020346. Molecules. 2025. PMID: 39860214 Free PMC article. Review.
Cited by
-
In Vitro Cytotoxicity Evaluation of Plastoquinone Analogues against Colorectal and Breast Cancers along with In Silico Insights.Pharmaceuticals (Basel). 2022 Oct 14;15(10):1266. doi: 10.3390/ph15101266. Pharmaceuticals (Basel). 2022. PMID: 36297378 Free PMC article.
-
Recruitment of hexahydroquinoline as anticancer scaffold targeting inhibition of wild and mutants EGFR (EGFRWT, EGFRT790M, and EGFRL858R).J Enzyme Inhib Med Chem. 2023 Dec;38(1):2241674. doi: 10.1080/14756366.2023.2241674. J Enzyme Inhib Med Chem. 2023. PMID: 37548154 Free PMC article.
-
Evaluation of anti-glioma effects of benzothiazoles as efficient apoptosis inducers and DNA cleaving agents.Mol Cell Biochem. 2023 May;478(5):1099-1108. doi: 10.1007/s11010-022-04580-4. Epub 2022 Oct 11. Mol Cell Biochem. 2023. PMID: 36219355
-
Comprehensive review of the repositioning of non-oncologic drugs for cancer immunotherapy.Med Oncol. 2024 Apr 23;41(5):122. doi: 10.1007/s12032-024-02368-8. Med Oncol. 2024. PMID: 38652344 Review.
-
Scaffold Hopping and Structural Modification of NSC 663284: Discovery of Potent (Non)Halogenated Aminobenzoquinones.Biomedicines. 2023 Dec 24;12(1):50. doi: 10.3390/biomedicines12010050. Biomedicines. 2023. PMID: 38255157 Free PMC article.
References
-
- Gewirtz D.A., Bristol M.L., Yalowich J.C. Toxicity issues in cancer drug development. Curr. Opin. Investig. Drugs. 2010;11:612–614. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

