Design and Activity of Novel Oxadiazole Based Compounds That Target Poly(ADP-ribose) Polymerase
- PMID: 35163965
- PMCID: PMC8839658
- DOI: 10.3390/molecules27030703
Design and Activity of Novel Oxadiazole Based Compounds That Target Poly(ADP-ribose) Polymerase
Abstract
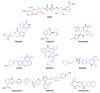
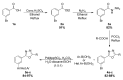
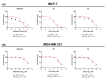
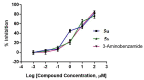
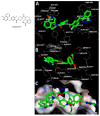
Novel PARP inhibitors with selective mode-of-action have been approved for clinical use. Herein, oxadiazole based ligands that are predicted to target PARP-1 have been synthesized and screened for the loss of cell viability in mammary carcinoma cells, wherein seven compounds were observed to possess significant IC50 values in the range of 1.4 to 25 µM. Furthermore, compound 5u, inhibited the viability of MCF-7 cells with an IC50 value of 1.4µM, when compared to Olaparib (IC50 = 3.2 µM). Compound 5s also decreased cell viability in MCF-7 and MDA-MB-231 cells with IC50 values of 15.3 and 19.2 µM, respectively. Treatment of MCF-7 cells with compounds 5u and 5s produced PARP cleavage, H2AX phosphorylation and CASPASE-3 activation comparable to that observed with Olaparib. Compounds 5u and 5s also decreased foci-formation and 3D Matrigel growth of MCF-7 cells equivalent to or greater than that observed with Olaparib. Finally, in silico analysis demonstrated binding of compound 5s towardsthe catalytic site of PARP-1, indicating that these novel oxadiazoles synthesized herein may serve as exemplars for the development of new therapeutics in cancer.
Keywords: human breast cancer; nicotinamide; oxadiazole; poly(ADP-ribose) polymerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

