Identification of Novel Cannabinoid CB2 Receptor Agonists from Botanical Compounds and Preliminary Evaluation of Their Anti-Osteoporotic Effects
- PMID: 35163968
- PMCID: PMC8838898
- DOI: 10.3390/molecules27030702
Identification of Novel Cannabinoid CB2 Receptor Agonists from Botanical Compounds and Preliminary Evaluation of Their Anti-Osteoporotic Effects
Abstract
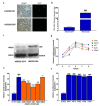
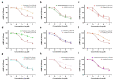
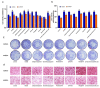
As cannabinoid CB2 receptors (CB2R) possess various pharmacological effects-including anti-epilepsy, analgesia, anti-inflammation, anti-fibrosis, and regulation of bone metabolism-without the psychoactive side effects induced by cannabinoid CB1R activation, they have become the focus of research and development of new target drugs in recent years. The present study was intended to (1) establish a double luciferase screening system for a CB2R modulator; (2) validate the agonistic activities of the screened compounds on CB2R by determining cAMP accumulation using HEK293 cells that are stably expressing CB2R; (3) predict the binding affinity between ligands and CB2 receptors and characterize the binding modes using molecular docking; (4) analyze the CB2 receptors-ligand complex stability, conformational behavior, and interaction using molecular dynamics; and (5) evaluate the regulatory effects of the screened compounds on bone metabolism in osteoblasts and osteoclasts. The results demonstrated that the screening system had good stability and was able to screen cannabinoid CB2R modulators from botanical compounds. Altogether, nine CB2R agonists were identified by screening from 69 botanical compounds, and these CB2R agonists exhibited remarkable inhibitory effects on cAMP accumulation and good affinity to CB2R, as evidenced by the molecular docking and molecular dynamics. Five of the nine CB2R agonists could stimulate osteoblastic bone formation and inhibit osteoclastic bone resorption. All these findings may provide useful clues for the development of novel anti-osteoporotic drugs and help elucidate the mechanism underlying the biological activities of CB2R agonists identified from the botanical materials.
Keywords: cannabinoid CB2 receptor agonists; double luciferase screening system; molecular docking and dynamics; osteoblast; osteoclast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Selective Cannabinoid 2 Receptor Agonists as Potential Therapeutic Drugs for the Treatment of Endotoxin-Induced Uveitis.Molecules. 2019 Sep 13;24(18):3338. doi: 10.3390/molecules24183338. Molecules. 2019. PMID: 31540271 Free PMC article.
-
Ligand-based virtual screening identifies a family of selective cannabinoid receptor 2 agonists.Bioorg Med Chem. 2015 Jan 1;23(1):241-63. doi: 10.1016/j.bmc.2014.11.002. Epub 2014 Nov 8. Bioorg Med Chem. 2015. PMID: 25487422 Free PMC article.
-
New Pyridone-Based Derivatives as Cannabinoid Receptor Type 2 Agonists.Int J Mol Sci. 2021 Oct 18;22(20):11212. doi: 10.3390/ijms222011212. Int J Mol Sci. 2021. PMID: 34681877 Free PMC article.
-
Pharmacological potential of JWH133, a cannabinoid type 2 receptor agonist in neurodegenerative, neurodevelopmental and neuropsychiatric diseases.Eur J Pharmacol. 2021 Oct 15;909:174398. doi: 10.1016/j.ejphar.2021.174398. Epub 2021 Jul 29. Eur J Pharmacol. 2021. PMID: 34332924 Review.
-
Patent review of cannabinoid receptor type 2 (CB2R) modulators (2016-present).Expert Opin Ther Pat. 2024 Aug;34(8):665-700. doi: 10.1080/13543776.2024.2368745. Epub 2024 Jul 2. Expert Opin Ther Pat. 2024. PMID: 38886185 Review.
Cited by
-
Exploring the therapeutic potential of natural compounds modulating the endocannabinoid system in various diseases and disorders: review.Pharmacol Rep. 2023 Dec;75(6):1410-1444. doi: 10.1007/s43440-023-00544-7. Epub 2023 Oct 31. Pharmacol Rep. 2023. PMID: 37906390 Review.
-
Cannabinoid Receptor Type 2 Agonist, GW405833, Reduced the Impacts of MDA-MB-231 Breast Cancer Cells on Bone Cells.Cancer Med. 2025 Feb;14(4):e70709. doi: 10.1002/cam4.70709. Cancer Med. 2025. PMID: 39980332 Free PMC article.
-
Cannabidiol Inhibits Inflammation Induced by Cutibacterium acnes-Derived Extracellular Vesicles via Activation of CB2 Receptor in Keratinocytes.J Inflamm Res. 2022 Aug 11;15:4573-4583. doi: 10.2147/JIR.S374692. eCollection 2022. J Inflamm Res. 2022. PMID: 35982758 Free PMC article.
-
Network Pharmacology, Molecular Docking, and Molecular Dynamic-Based Investigation on the Mechanism of Compound Chrysanthemum in the Treatment of Asthenopia.Comput Math Methods Med. 2022 Dec 30;2022:3444277. doi: 10.1155/2022/3444277. eCollection 2022. Comput Math Methods Med. 2022. PMID: 36619789 Free PMC article.
-
CB2 regulates oxidative stress and osteoclastogenesis through NOX1-dependent signaling pathway in titanium particle-induced osteolysis.Cell Death Discov. 2023 Dec 16;9(1):461. doi: 10.1038/s41420-023-01761-y. Cell Death Discov. 2023. PMID: 38104087 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

