The Bright Side of the Tiger: Autofluorescence Patterns in Aedes albopictus (Diptera, Culicidae) Male and Female Mosquitoes
- PMID: 35163978
- PMCID: PMC8839535
- DOI: 10.3390/molecules27030713
The Bright Side of the Tiger: Autofluorescence Patterns in Aedes albopictus (Diptera, Culicidae) Male and Female Mosquitoes
Abstract
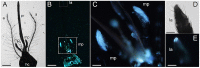
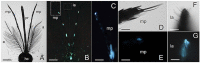
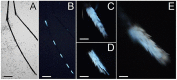
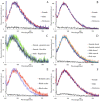
Light-based events in insects deserve increasing attention for various reasons. Besides their roles in inter- and intra-specific visual communication, with biological, ecological and taxonomical implications, optical properties are also promising tools for the monitoring of insect pests and disease vectors. Among these is the Asian tiger mosquito, Aedes albopictus, a global arbovirus vector. Here we have focused on the autofluorescence characterization of Ae. albopictus adults using a combined imaging and spectrofluorometric approach. Imaging has evidenced that autofluorescence rises from specific body compartments, such as the head appendages, and the abdominal and leg scales. Spectrofluorometry has demonstrated that emission consists of a main band in the 410-600 nm region. The changes in the maximum peak position, between 430 nm and 500 nm, and in the spectral width, dependent on the target structure, indicate the presence, at variable degrees, of different fluorophores, likely resilin, chitin and melanins. The aim of this work has been to provide initial evidence on the so far largely unexplored autofluorescence of Ae. albopictus, to furnish new perspectives for the set-up of species- and sex-specific investigation of biological functions as well as of strategies for in-flight direct detection and surveillance of mosquito vectors.
Keywords: antennae; chitin; imaging; maxillary palps; melanin; resilin; scales; sexual dimorphism; spectrofluorometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Detection of the Invasive Mosquito Species Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Portugal.Int J Environ Res Public Health. 2018 Apr 21;15(4):820. doi: 10.3390/ijerph15040820. Int J Environ Res Public Health. 2018. PMID: 29690531 Free PMC article.
-
Deciphering the olfactory repertoire of the tiger mosquito Aedes albopictus.BMC Genomics. 2017 Oct 11;18(1):770. doi: 10.1186/s12864-017-4144-1. BMC Genomics. 2017. PMID: 29020917 Free PMC article.
-
Monitoring of alien mosquitoes of the genus Aedes (Diptera: Culicidae) in Austria.Parasitol Res. 2019 May;118(5):1633-1638. doi: 10.1007/s00436-019-06287-w. Epub 2019 Mar 16. Parasitol Res. 2019. PMID: 30877440 Free PMC article.
-
Globe-Trotting Aedes aegypti and Aedes albopictus: Risk Factors for Arbovirus Pandemics.Vector Borne Zoonotic Dis. 2020 Feb;20(2):71-81. doi: 10.1089/vbz.2019.2486. Epub 2019 Sep 26. Vector Borne Zoonotic Dis. 2020. PMID: 31556813 Free PMC article. Review.
-
Aedes albopictus (Diptera: Culicidae) and Mosquito-Borne Viruses in the United States.J Med Entomol. 2016 Sep;53(5):1024-8. doi: 10.1093/jme/tjw025. Epub 2016 Apr 25. J Med Entomol. 2016. PMID: 27113107 Review.
Cited by
-
Autofluorescent Biomolecules in Diptera: From Structure to Metabolism and Behavior.Molecules. 2022 Jul 12;27(14):4458. doi: 10.3390/molecules27144458. Molecules. 2022. PMID: 35889334 Free PMC article. Review.
-
Imaging and spectral analysis of autofluorescence patterns in larval head structures of mosquito vectors.Eur J Histochem. 2022 Sep 20;66(4):3462. doi: 10.4081/ejh.2022.3462. Eur J Histochem. 2022. PMID: 36128772 Free PMC article.
-
Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus.Int J Mol Sci. 2023 Dec 23;25(1):245. doi: 10.3390/ijms25010245. Int J Mol Sci. 2023. PMID: 38203417 Free PMC article.
-
The origin of black and white coloration of the Asian tiger mosquito Aedes albopictus (Diptera: Culicidae).Beilstein J Nanotechnol. 2023 Apr 17;14:496-508. doi: 10.3762/bjnano.14.41. eCollection 2023. Beilstein J Nanotechnol. 2023. PMID: 37123532 Free PMC article.
References
-
- Udenfriend S. In: Fluorescence Assay in Biology and Medicine. 1st ed. Horecker B., Kaplan N.O., Marmur J., Scheraga H.A., editors. Vol. 2. Academic Press; New York, NY, USA: London, UK: 1969. pp. 195–404.
-
- Croce A.C. Light and autofluorescence, multitasking features in living organisms. Photochem. 2021;1:67–125. doi: 10.3390/photochem1020007. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

