Laser-Induced Generation of Hydrogen in Water by Using Graphene Target
- PMID: 35163983
- PMCID: PMC8839270
- DOI: 10.3390/molecules27030718
Laser-Induced Generation of Hydrogen in Water by Using Graphene Target
Abstract
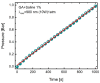
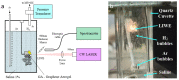
A new method of hydrogen generation from water, by irradiation with CW infrared laser diode of graphene scaffold immersed in solution, is reported. Hydrogen production was extremely efficient upon admixing NaCl into water. The efficiency of hydrogen production increased exponentially with laser power. It was shown that hydrogen production was highly efficient when the intense white light emission induced by laser irradiation of graphene foam was occurring. The mechanism of laser-induced dissociation of water is discussed. It was found that hydrogen production was extremely high, at about 80%, and assisted by a small emission of O2, CO and CO2 gases.
Keywords: graphene foam; hydrogen generation; laser irradiation; white light emission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Abuadala A., Dincer I. A review on biomass-based hydrogen production and potential applications. Int. J. Energy Res. 2012;36:415–455. doi: 10.1002/er.1939. - DOI
-
- Mujeebu M.A. Hydrogen and syngas production by superadiabatic combustion—A review. Appl. Energy. 2016;173:210–224. doi: 10.1016/j.apenergy.2016.04.018. - DOI
-
- Kalinci Y., Hepbasli A., Dincer I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy. 2009;34:8799–8817. doi: 10.1016/j.ijhydene.2009.08.078. - DOI
-
- Rahimpour M.R., Jafari M., Iranshahi D. Progress in catalytic naphtha reforming process: A review. Appl. Energy. 2013;109:79–93. doi: 10.1016/j.apenergy.2013.03.080. - DOI
-
- Trane R., Dahl S., Skjøth-Rasmussen M.S., Jensen A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy. 2012;37:6447–6472. doi: 10.1016/j.ijhydene.2012.01.023. - DOI
LinkOut - more resources
Full Text Sources

