Flavanols from Nature: A Phytochemistry and Biological Activity Review
- PMID: 35163984
- PMCID: PMC8838462
- DOI: 10.3390/molecules27030719
Flavanols from Nature: A Phytochemistry and Biological Activity Review
Abstract
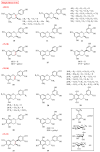
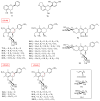
Flavanols, a common class of secondary plant metabolites, exhibit several beneficial health properties by acting as antioxidant, anticarcinogen, cardioprotective, anti-microbial, anti-viral, and neuroprotective agents. Furthermore, some flavanols are considered functional ingredients in dairy products. Based on their structural features and health-promoting functions, flavanols have gained the attention of pharmacologists and botanists worldwide. This review collects and summarizes 121 flavanols comprising four categories: flavan-3-ols, flavan-4-ols, isoflavan-4-ols, and flavan-3,4-ols. The research of the various structural features and pharmacological activities of flavanols and their derivatives aims to lay the groundwork for subsequent research and expect to provide mentality and inspiration for the research. The current study provides a starting point for further research and development.
Keywords: biological activities; flavanols; flavonoids; natural products.
Conflict of interest statement
The authors have no competing interests.
Figures



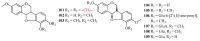
References
-
- Knaze V., Zamora R.R., Lujan B.L. Intake estimation of total and individual flavan-3-ols, proanthocyanidins and theaflavins, their food sources and determinants in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br. J. Nutr. 2012;108:1095–1108. doi: 10.1017/S0007114511006386. - DOI - PubMed
-
- Zhou Q., Jian Y., Yi P., Sun J., Wang W. A comprehensive review on Pronephrium penangianum. Isr. J. Chem. 2019;59:371–377. doi: 10.1002/ijch.201800141. - DOI
-
- Diana J., O’Leary M., Philip W., Lindberg M.B., Semple S.J. Phytochemistry and bioactivity of Acacia sensu stricto (Fabaceae: Mimosoideae) Phytochem. Rev. 2018;18:129–172.
-
- Cho J.Y., Moon J.H., Eun J.B., Chung S.J., Park K.H. Isolation and characterization of 3(Z)-dodecenedioic acid as an antibacterial substance from Hovenia dulcis thunb. Food Sci. Biotechnol. 2004;13:46–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

