The Structural and Optical Properties of 1,2,4-Triazolo[4,3- a]pyridine-3-amine
- PMID: 35163987
- PMCID: PMC8838196
- DOI: 10.3390/molecules27030721
The Structural and Optical Properties of 1,2,4-Triazolo[4,3- a]pyridine-3-amine
Abstract
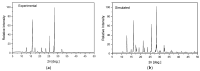
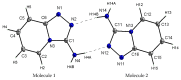
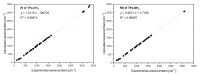
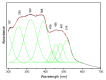
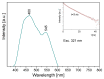
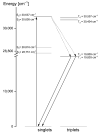
The structural and spectroscopic properties of a new triazolopyridine derivative (1,2,4-triazolo[4,3-a]pyridin-3-amine) are described in this paper. Its FTIR spectrum was recorded in the 100-4000 cm-1 range and its FT-Raman spectrum in the range 80-4000 cm-1. The molecular structure and vibrational spectra were analyzed using the B3LYP/6-311G(2d,2p) approach and the GAUSSIAN 16W program. The assignment of the observed bands to the respective normal modes was proposed on the basis of PED calculations. XRD studies revealed that the studied compound crystallizes in the centrosymmetric monoclinic space group P21/n with eight molecules per unit cell. However, the asymmetric unit contains two 1,2,4-triazolo[4,3-a]pyridin-3-amine molecules linked via N-H⋯N hydrogen bonds with a R22(8) graph. The stability of the studied molecule was considered using NBO analysis. Electron absorption and the luminescence spectra were measured and discussed in terms of the calculated singlet, triplet, HOMO and LUMO electron energies. The Stokes shifts derived from the optical spectra were equal to 9410 cm-1 for the triazole ring and 7625 cm-1 for the pyridine ring.
Keywords: 1,2,4-triazolo[4,3-a]pyridin-3-amine; XRD studies; molecular structure; spectroscopic analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Physicochemical Characterization of the Loganic Acid-IR, Raman, UV-Vis and Luminescence Spectra Analyzed in Terms of Quantum Chemical DFT Approach.Molecules. 2021 Nov 20;26(22):7027. doi: 10.3390/molecules26227027. Molecules. 2021. PMID: 34834118 Free PMC article.
-
Spectroscopic studies (FTIR, FT-Raman and UV-Visible), normal coordinate analysis, NBO analysis, first order hyper polarizability, HOMO and LUMO analysis of (1R)-N-(Prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-amine molecule by ab initio HF and density functional methods.Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:394-403. doi: 10.1016/j.saa.2013.10.093. Epub 2013 Oct 30. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24280302
-
X-ray crystal structure, vibrational spectra and DFT calculations of 3-chloro-7-azaindole: a case of dual N-H⋯N hydrogen bonds in dimers.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt B:405-15. doi: 10.1016/j.saa.2014.09.050. Epub 2014 Sep 28. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25315872
-
Spectroscopic and optical properties of 1,2,4-triazolo[4,3-a]pyridin-3(2H)-one as a component of herbicides.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Dec 15;303:123141. doi: 10.1016/j.saa.2023.123141. Epub 2023 Jul 13. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 37481842
-
Structural and vibrational properties of imidazo[4,5-c]pyridine, a structural unit in natural products.J Nat Prod. 2013 Sep 27;76(9):1637-46. doi: 10.1021/np400293j. Epub 2013 Sep 6. J Nat Prod. 2013. PMID: 24070053
Cited by
-
Exploring the Absorption Mechanisms of Imidazolium-Based Ionic Liquids to Epigallocatechin Gallate.Int J Mol Sci. 2022 Oct 20;23(20):12600. doi: 10.3390/ijms232012600. Int J Mol Sci. 2022. PMID: 36293456 Free PMC article.
References
-
- Zhai Z.-W., Shi Y.-X., Yang M.-Y., Zhao W., Sun Z.-H., Weng J.-Q., Tan C.-X., Liu X.-H., Li B.-J., Zhang Y.G. Microwave Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-triazolo[4,3-a] pyridine Moiety. Lett. Drug Des. Discov. 2016;13:521–525. doi: 10.2174/157018081306160618181757. - DOI
-
- Wang Q., Zhai Z., Sun Z., Liu X., Tan C., Weng J. Synthesis, Crystal Structure and Antifungal Activity of 8-Chloro-3-((4-chlorobenzyl)-thio)[1,2,4]triazolo[4,3-a] pyridine. Chin. J. Struct. Chem. 2016;35:651–655. doi: 10.1016/j.cjche.2016.01.002. - DOI
-
- Xu F.Z., Shao J.H., Zhu Y.Y., Liu L.W., Zhao Y.H., Shan W.L., Xue W. Synthesis, antifungal and insecticidal activity of novel[1,2,4]triazolo[4, 3-a] pyridine derivatives containing a sulfide substructure. Chem. Pap. 2017;71:729–739. doi: 10.1007/s11696-016-0006-6. - DOI
-
- Sadana A.K., Mirza Y., Aneja K.R., Prakash O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo [4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003;38:533–536. doi: 10.1016/S0223-5234(03)00061-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources

