An Improved Approach for Practical Synthesis of 5-Hydroxymethyl-2'-deoxycytidine (5hmdC) Phosphoramidite and Triphosphate
- PMID: 35164012
- PMCID: PMC8839764
- DOI: 10.3390/molecules27030749
An Improved Approach for Practical Synthesis of 5-Hydroxymethyl-2'-deoxycytidine (5hmdC) Phosphoramidite and Triphosphate
Abstract
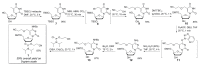
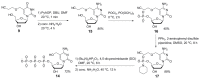
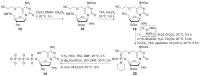
5-Hydroxymethyl-2'-deoxycytidine (5hmdC) phosphoramidite and triphosphate are important building blocks in 5hmdC-containing DNA synthesis for epigenetic studies. However, efficient and practical methods for the synthesis of these compounds are still limited. The current research provides an intensively improved synthetic method that enables the preparation of commercially available cyanoethyl-protected 5hmdC phosphoramidite with an overall yield of 39% on 5 g scale. On the basis of facile and efficient accesses to cyanoethyl protected-5hmdU and 5hmdC intermediates, two efficient synthetic routes for 5hmdC triphosphate were also developed.
Keywords: 5-hydroxymethyl-2′-deoxycytidine (5hmdC); P(V)-N activation; cyanoethyl ether; phosphoramidite; triphosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Associations of urinary 5-methyl-2'-deoxycytidine and 5-hydroxymethyl-2'-deoxycytidine with phthalate exposure and semen quality in 562 Chinese adult men.Environ Int. 2016 Sep;94:583-590. doi: 10.1016/j.envint.2016.06.020. Epub 2016 Jun 24. Environ Int. 2016. PMID: 27346742
-
A DNA adductome analysis revealed a reduction in the global level of C5-hydroxymethyl-2'-deoxycytidine in the non-tumoral upper urinary tract mucosa of urothelial carcinoma patients.Genes Environ. 2021 Dec 2;43(1):52. doi: 10.1186/s41021-021-00228-9. Genes Environ. 2021. PMID: 34852853 Free PMC article.
-
Preparation of DNA containing 5-hydroxymethyl-2'-deoxycytidine modification through phosphoramidites with TBDMS as 5-hydroxymethyl protecting group.Curr Protoc Nucleic Acid Chem. 2011 Dec;Chapter 4:Unit 4.47.1-18. doi: 10.1002/0471142700.nc0447s47. Curr Protoc Nucleic Acid Chem. 2011. PMID: 22147420 Free PMC article.
-
New approaches to synthesis of stereospecific sphingomyelin.Chem Phys Lipids. 1999 Nov;102(1-2):3-12. doi: 10.1016/s0009-3084(99)00070-5. Chem Phys Lipids. 1999. PMID: 11001556 Review.
-
Synthesis of 8-oxoguanosine phosphoramidite and its incorporation into Oligoribonucleotides.Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:4.58.1-10. doi: 10.1002/0471142700.nc0458s56. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25606978 Review.
Cited by
-
Convenient syntheses of isotopically labeled pyrimidine 2'-deoxynucleosides and their 5-hydroxy oxidation products.Nucleosides Nucleotides Nucleic Acids. 2024 Dec 9:1-23. doi: 10.1080/15257770.2024.2437038. Online ahead of print. Nucleosides Nucleotides Nucleic Acids. 2024. PMID: 39652906
References
-
- Tahiliani M., Koh K.P., Shen Y.-H., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

