An Improved Approach for Practical Synthesis of 5-Hydroxymethyl-2'-deoxycytidine (5hmdC) Phosphoramidite and Triphosphate
- PMID: 35164012
- PMCID: PMC8839764
- DOI: 10.3390/molecules27030749
An Improved Approach for Practical Synthesis of 5-Hydroxymethyl-2'-deoxycytidine (5hmdC) Phosphoramidite and Triphosphate
Abstract
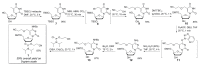
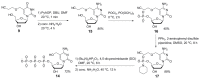
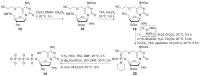
5-Hydroxymethyl-2'-deoxycytidine (5hmdC) phosphoramidite and triphosphate are important building blocks in 5hmdC-containing DNA synthesis for epigenetic studies. However, efficient and practical methods for the synthesis of these compounds are still limited. The current research provides an intensively improved synthetic method that enables the preparation of commercially available cyanoethyl-protected 5hmdC phosphoramidite with an overall yield of 39% on 5 g scale. On the basis of facile and efficient accesses to cyanoethyl protected-5hmdU and 5hmdC intermediates, two efficient synthetic routes for 5hmdC triphosphate were also developed.
Keywords: 5-hydroxymethyl-2′-deoxycytidine (5hmdC); P(V)-N activation; cyanoethyl ether; phosphoramidite; triphosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tahiliani M., Koh K.P., Shen Y.-H., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

