Antimicrobial, Cytotoxic and Mutagenic Activity of Gemini QAS Derivatives of 1,4:3,6-Dianhydro-l-iditol
- PMID: 35164023
- PMCID: PMC8838521
- DOI: 10.3390/molecules27030757
Antimicrobial, Cytotoxic and Mutagenic Activity of Gemini QAS Derivatives of 1,4:3,6-Dianhydro-l-iditol
Abstract
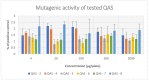
A series of quaternary diammonium salts derivatives of 1,4:3,6-dianhydro-l-iditol were synthesized, using isommanide (1,4:3,6-dianhydro-d-mannitol) as a starting material. Both aromatic (pyridine, 4-(N,N-dimethylamino)pyridine (DMAP), (3-carboxamide)pyridine; N-methylimidazole) and aliphatic (trimethylamine, N,N-dimethylhexylamine, N,N-dimethyloctylamine, N,N-dimethyldecylamine) amines were used, giving eight gemini quaternary ammonium salts (QAS). All salts were tested for their antimicrobial activity against yeasts, Candida albicans and Candida glabrata, as well as bacterial Staphylococcus aureus and Escherichia coli reference strains. Moreover, antibacterial activity against 20 isolates of S. aureus collected from patients with skin and soft tissue infections (n = 8) and strains derived from subclinical bovine mastitis milk samples (n = 12) were evaluated. Two QAS with octyl and decyl residues exhibited antimicrobial activity, whereas those with two decyl residues proved to be the most active against the tested pathogens, with MIC of 16-32, 32, and 8 µg/mL for yeast, E. coli, and S. aureus reference and clinical strains, respectively. Only QAS with decyl residues proved to be cytotoxic in MTT assay against human keratinocytes (HaCaT), IC50 12.8 ± 1.2 μg/mL. Ames test was used to assess the mutagenic potential of QAS, and none of them showed mutagenic activity in the concentration range 4-2000 µg/plate.
Keywords: 1,4:3,6-dianhydro-d-mannitol; antimicrobial activity; gemini; mutagenicity; quaternary ammonium salts.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Newly synthesized surfactants as antimicrobial and anti-adhesion agents.Colloids Surf B Biointerfaces. 2024 Jul;239:113932. doi: 10.1016/j.colsurfb.2024.113932. Epub 2024 May 1. Colloids Surf B Biointerfaces. 2024. PMID: 38749165
-
Antifungal activity of gemini quaternary ammonium salts.Microbiol Res. 2013 Dec 14;168(10):630-8. doi: 10.1016/j.micres.2013.06.001. Epub 2013 Jul 1. Microbiol Res. 2013. PMID: 23827647
-
Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers.Molecules. 2017 Oct 25;22(11):1810. doi: 10.3390/molecules22111810. Molecules. 2017. PMID: 29068383 Free PMC article.
-
Activity of gemini quaternary ammonium salts against microorganisms.Appl Microbiol Biotechnol. 2019 Jan;103(2):625-632. doi: 10.1007/s00253-018-9523-2. Epub 2018 Nov 20. Appl Microbiol Biotechnol. 2019. PMID: 30460534 Review.
-
Micellization and other associations of amphiphilic antimicrobial quaternary ammonium salts in aqueous solutions.Pharmazie. 1996 Mar;51(3):135-44. Pharmazie. 1996. PMID: 8900863 Review.
Cited by
-
Biocidal and antibiofilm activities of arginine-based surfactants against Candida isolates.Amino Acids. 2023 Sep;55(9):1083-1102. doi: 10.1007/s00726-023-03296-z. Epub 2023 Jun 29. Amino Acids. 2023. PMID: 37382761
-
Advances in the Synthesis of Biologically Active Quaternary Ammonium Compounds.Int J Mol Sci. 2024 Apr 24;25(9):4649. doi: 10.3390/ijms25094649. Int J Mol Sci. 2024. PMID: 38731869 Free PMC article. Review.
-
Comparative putative metabolites profiling of Tachypleus gigas and Carcinoscorpius rotundicauda hemocytes stimulated with lipopolysaccharide.Sci Rep. 2024 Feb 17;14(1):3968. doi: 10.1038/s41598-024-54279-3. Sci Rep. 2024. PMID: 38368470 Free PMC article. Review.
-
Staphylococcus aureus in Bovine Mastitis: A Narrative Review of Prevalence, Antimicrobial Resistance, and Advances in Detection Strategies.Antibiotics (Basel). 2025 Aug 8;14(8):810. doi: 10.3390/antibiotics14080810. Antibiotics (Basel). 2025. PMID: 40868004 Free PMC article. Review.
References
-
- Pang J., Wang A., Zheng M., Zhang Y., Huang Y., Chen X., Zhang T. Catalytic conversion of cellulose to hexitols with mesoporous carbon supported Ni-based bimetallic catalysts. Green Chem. 2012;14:614–617. doi: 10.1039/c2gc16364k. - DOI
-
- Liu Y., Chen L., Wang T., Xu Y., Zhang Q., Ma L., Liao Y., Shi N. Direct conversion of cellulose into C 6 alditols over Ru/C combined with H + -released boron phosphate in an aqueous phase. RSC Adv. 2014;4:52402–52409. doi: 10.1039/C4RA10834E. - DOI
-
- Luo Y., Li Z., Li X., Liu X., Fan J., Clark J.H., Hu C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today. 2019;319:14–24. doi: 10.1016/j.cattod.2018.06.042. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

