Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions
- PMID: 35164036
- PMCID: PMC8839487
- DOI: 10.3390/molecules27030772
Visible-Light-Induced, Graphene Oxide-Promoted C3-Chalcogenylation of Indoles Strategy under Transition-Metal-Free Conditions
Abstract
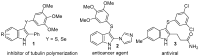
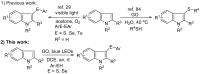
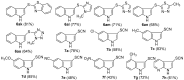
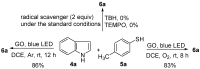
An efficient and general method for the synthesis of 3-sulfenylindoles and 3-selenylindoles employing visible-light irradiation with graphene oxide as a promoter at room temperature has been achieved. The reaction features are high yields, simple operation, metal-free and iodine-free conditions, an easy-to-handle oxidant, and gram-scalable synthesis. This simple protocol allows one to access a wide range of 3-arylthioindoles, 3-arylselenylindoles, and even 3-thiocyanatoindoles with good to excellent yields.
Keywords: graphene oxide; indole; selenylindole; sulfenylindole; visible-light.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
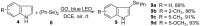
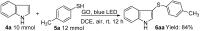

References
-
- Ninomiya M., Garud D.R., Koketsu M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011;255:2968–2990. doi: 10.1016/j.ccr.2011.07.009. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

