New 1,2,3-Triazoles from (R)-Carvone: Synthesis, DFT Mechanistic Study and In Vitro Cytotoxic Evaluation
- PMID: 35164037
- PMCID: PMC8839216
- DOI: 10.3390/molecules27030769
New 1,2,3-Triazoles from (R)-Carvone: Synthesis, DFT Mechanistic Study and In Vitro Cytotoxic Evaluation
Abstract
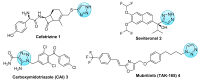
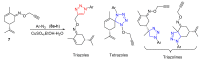
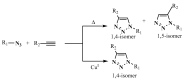
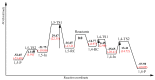
Aseries of novel 1,4-disubstituted 1,2,3-triazoles were synthesized from an (R)-carvone terminal alkyne derivative via a Cu (I)-catalyzed azide-alkyne cycloaddition reaction using CuSO4,5H2O as the copper (II) source and sodium ascorbate as a reducing agent which reduces Cu (II) into Cu (I). All the newly synthesized 1,2,3-triazoles 9a-h were fully identified on the basis of their HRMS and NMR spectral data and then evaluated for their cell growth inhibition potential by MTS assay against HT-1080 fibrosarcoma, A-549 lung carcinoma, and two breast adenocarcinoma (MCF-7 and MDA-MB-231) cell lines. Compound 9d showed notable cytotoxic effects against the HT-1080 and MCF-7 cells with IC50 values of 25.77 and 27.89 µM, respectively, while compound 9c displayed significant activity against MCF-7 cells with an IC50 value of 25.03 µM. Density functional calculations at the B3LYP/6-31G* level of theory were used to confirm the high reactivity of the terminal alkyne as a dipolarophile. Quantum calculations were also used to investigate the mechanism of both the uncatalyzed and copper (I)-catalyzed azide-alkyne cycloaddition reaction (CuAAC). The catalyzed reaction gives complete regioselectivity via a stepwise mechanism streamlining experimental observations. The calculated free-energy barriers 4.33 kcal/mol and 29.35 kcal/mol for the 1,4- and 1,5-regioisomers, respectively, explain the marked regioselectivity of the CuAAC reaction.
Keywords: (R)-carvone; 1,2,3-triazole; DFT calculations; cytotoxic activity; regioselectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
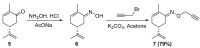

Similar articles
-
Thiazolidinone-linked1,2,3-triazoles with monoterpenic skeleton as new potential anticancer agents: Design, synthesis and molecular docking studies.Bioorg Chem. 2021 Oct;115:105184. doi: 10.1016/j.bioorg.2021.105184. Epub 2021 Jul 20. Bioorg Chem. 2021. PMID: 34333421 Review.
-
Newly synthesized (R)-carvone-derived 1,2,3-triazoles: structural, mechanistic, cytotoxic and molecular docking studies.J Biomol Struct Dyn. 2022 Oct;40(16):7205-7217. doi: 10.1080/07391102.2021.1894984. Epub 2021 Mar 15. J Biomol Struct Dyn. 2022. PMID: 33719863
-
New 1,2,3-Triazole-Containing Hybrids as Antitumor Candidates: Design, Click Reaction Synthesis, DFT Calculations, and Molecular Docking Study.Molecules. 2021 Jan 29;26(3):708. doi: 10.3390/molecules26030708. Molecules. 2021. PMID: 33573040 Free PMC article.
-
Hybrids of thiazolidinone with 1,2,3-triazole derivatives: design, synthesis, biological evaluation, in silico studies, molecular docking, molecular dynamics simulations, and ADMET profiling.J Biomol Struct Dyn. 2023;41(21):11987-11999. doi: 10.1080/07391102.2022.2164357. Epub 2023 Jan 9. J Biomol Struct Dyn. 2023. PMID: 36617941
-
Computational studies on the regioselectivity of metal-catalyzed synthesis of 1,2,3 triazoles via click reaction: a review.J Mol Model. 2015 Oct;21(10):264. doi: 10.1007/s00894-015-2810-2. Epub 2015 Sep 18. J Mol Model. 2015. Retraction in: J Mol Model. 2019 Mar 7;25(4):86. doi: 10.1007/s00894-019-3978-7. PMID: 26385849 Retracted. Review.
Cited by
-
Synthesis, characterization, molecular docking, and anticancer activities of new 1,3,4-oxadiazole-5-fluorocytosine hybrid derivatives.J Mol Struct. 2022 Sep 9:134135. doi: 10.1016/j.molstruc.2022.134135. Online ahead of print. J Mol Struct. 2022. PMID: 36101881 Free PMC article.
-
New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization.J Enzyme Inhib Med Chem. 2022 Dec;37(1):1346-1363. doi: 10.1080/14756366.2022.2072308. J Enzyme Inhib Med Chem. 2022. PMID: 35548854 Free PMC article.
-
Thiazolidinone-linked-1,2,3-triazoles with (R)-Carvone as new potential anticancer agents.Future Med Chem. 2024;16(14):1449-1464. doi: 10.1080/17568919.2024.2351287. Epub 2024 May 30. Future Med Chem. 2024. PMID: 39190475 Free PMC article.
-
Novel Bis-1,2,3-triazole-thiazolidinone hybrid as anticancer agents that induce apoptosis and molecular modeling study.Future Med Chem. 2024;16(21):2193-2210. doi: 10.1080/17568919.2024.2394019. Epub 2024 Oct 10. Future Med Chem. 2024. PMID: 39387360
-
Synthesis of Novel Fluoro Phenyl Triazoles Via Click Chemistry with or without Microwave Irradiation and their Evaluation as Anti-proliferative Agents in SiHa Cells.Curr Org Synth. 2024;21(4):559-570. doi: 10.2174/1570179420666230420084000. Curr Org Synth. 2024. PMID: 37078356
References
-
- Liqiao S., Rui H., Yanhong W., Ying L., Ziwen Y., Shaoyong K., Shi L., Hu R., Wei Y., Liang Y., et al. Anthranilic Acid-Based Diamides Derivatives Incorporating Aryl-Isoxazoline Pharmacophore as Potential Anticancer Agents: Design, Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2012;54:549–556. doi: 10.1016/j.ejmech.2012.06.001. - DOI - PubMed
-
- Govind P. Malnutrition leading to cancer by some environmental hazard. [(accessed on 1 September 2021)];IJRAP. 2010 1:287–291. Available online: www.ijrap.Net.
-
- Yaremenko I.A., Syroeshkin M.A., Levitsky D.O., Fleury F., Terent’ev A.O. Cyclic Peroxides as Promising Anticancer Agents: In Vitro Cytotoxicity Study of Synthetic Ozonides and Tetraoxanes on Human Prostate Cancer Cell Lines. Med. Chem. Res. 2017;26:170–179. doi: 10.1007/s00044-016-1736-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

