Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds
- PMID: 35164041
- PMCID: PMC8838714
- DOI: 10.3390/molecules27030777
Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds
Abstract
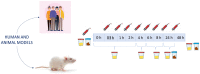
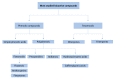
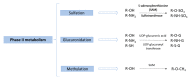
The study of the bioavailability of bioactive compounds is a fundamental step for the development of applications based on them, such as nutraceuticals, functional foods or cosmeceuticals. It is well-known that these compounds can undergo metabolic reactions before reaching therapeutic targets, which may also affect their bioactivity and possible applications. All recent studies that have focused on bioavailability and metabolism of phenolic and terpenoid compounds have been developed because of the advances in analytical chemistry and metabolomics approaches. The purpose of this review is to show the role of analytical chemistry and metabolomics in this field of knowledge. In this context, the different steps of the analytical chemistry workflow (design study, sample treatment, analytical techniques and data processing) applied in bioavailability and metabolism in vivo studies are detailed, as well as the most relevant results obtained from them.
Keywords: analytical chemistry; bioactive compounds; bioavailability; chromatography; mass spectrometry; metabolism; metabolomics; phenolic compounds; phytochemicals; untargeted.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hosseinzadeh S., Jafarikukhdan A., Hosseini A., Armand R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015;06:635–642. doi: 10.4236/ijcm.2015.69084. - DOI
-
- Singh R. Medicinal Plants: A Review. J. Plant Sci. 2015;3:50–55. doi: 10.11648/j.jps.s.2015030101.18. - DOI
-
- Cádiz-Gurrea M.d.l.L., Villegas-Aguilar M.d.C., Leyva-Jiménez F.J., Pimentel-Moral S., Fernández-Ochoa Á., Alañón M.E., Segura-Carretero A. Revalorization of bioactive compounds from tropical fruit by-products and industrial applications by means of sustainable approaches. Food Res. Int. 2020;138:109786. doi: 10.1016/j.foodres.2020.109786. - DOI - PubMed
-
- Mitra S., Naskar N., Chaudhuri P. A review on potential bioactive phytochemicals for novel therapeutic applications with special emphasis on mangrove species. Phytomedicine Plus. 2021;1:100107. doi: 10.1016/j.phyplu.2021.100107. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

