MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells
- PMID: 35164046
- PMCID: PMC8837934
- DOI: 10.3390/molecules27030782
MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells
Abstract
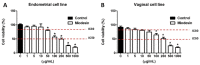
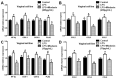
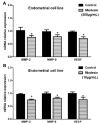
Endometriosis presents high prevalence and its physiopathology involves hyperactivation of endometrial and vaginal cells, especially by bacteria. The disease has no cure and therapies aiming to inhibit its development are highly desirable. Therefore, this study investigated whether MiodesinTM (10 µg/mL = IC80; 200 µg/mL = IC50), a natural compound constituted by Uncaria tomentosa, Endopleura uchi, and astaxanthin, could exert anti-inflammatory and anti-proliferative effects against Lipopolysaccharides (LPS) stimulation in endometrial and Candida albicans vaginal cell lines. VK2 E6/E7 (vaginal) and KLE (epithelial) cell lines were stimulated with Candida albicans (1 × 107 to 5 × 107/mL) and LPS (1 μg/mL), respectively. MiodesinTM inhibited mRNA expression for Nuclear factor kappa B (NF-κB), ciclo-oxigenase 1 (COX-1), and phospholipase A2 (PLA2), beyond the C-C motif chemokine ligand 2 (CCL2), CCL3, and CCL5 in VK2 E6/E7 cells (p < 0.05). In addition, the inhibitory effects of both doses of MiodesinTM (10 µg/mL and 200 µg/mL) resulted in reduced secretion of interleukin-1β (IL-1β), IL-6, IL-8, tumor necrosis factor α (TNF-α) (24 h, 48 h, and 72 h) and CCL2, CCL3, and CLL5 (p < 0.05) by VK2 E6/E7 cells. In the same way, COX-1 MiodesinTM inhibited LPS-induced hyperactivation of KLE cells, as demonstrated by reduced secretion of IL-1β, IL-6, IL-8, TNF-α (24 h, 48 h, and 72 h) and CCL2, CCL3, and CLL5 (p < 0.05). Furthermore, MiodesinTM also inhibited mRNA expression and secretion of matrix metalloproteinase-2 (MMP-2), MMP-9, and vascular endothelial growth factor (VEGF), which are key regulators of invasion of endometrial cells. Thus, the study concludes that MiodesinTM presents beneficial effects in the context of endometriosis, positively affecting the inflammatory and proliferative response.
Keywords: MiodesinTM; anti-inflammatory; chemokines; endometriosis; leiomyoma.
Conflict of interest statement
All authors disclose any influence of companies or manufacturers in the present study. In addition, all authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Figures


References
-
- Murji A., Bedaiwy M., Singh S.S., Bougie O., Committee C.R.S. Influence of Ethnicity on Clinical Presentation and Quality of Life in Women with Uterine Fibroids: Results from a Prospective Observational Registry. J. Obstet. Gynaecol. Can. 2020;42:726–733. doi: 10.1016/j.jogc.2019.10.031. - DOI - PubMed
-
- Yu H., Zhong Q., Xia Y., Li E., Wang S., Ren R. MicroRNA-2861 Targets STAT3 and MMP2 to Regulate the Proliferation and Apoptosis of Ectopic Endometrial Cells in Endometriosis. Die Pharm.-Int. J. Pharm. Sci. 2019;74:243–249. - PubMed
-
- Bostanci Durmus A., Dincer Cengiz S., Yılmaz H., Candar T., Gursoy A.Y., Sinem Caglar G. The Levels of Matrix Metalloproteinase-9 and Neutrophil Gelatinase-Associated Lipocalin in Different Stages of Endometriosis. J. Obstet. Gynaecol. 2019;39:991–995. doi: 10.1080/01443615.2019.1584889. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

