Different Schiff Bases-Structure, Importance and Classification
- PMID: 35164049
- PMCID: PMC8839460
- DOI: 10.3390/molecules27030787
Different Schiff Bases-Structure, Importance and Classification
Abstract
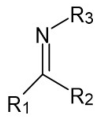
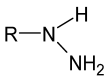
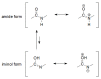
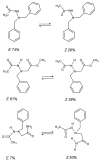
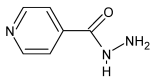
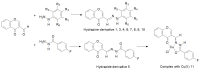
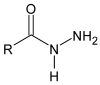
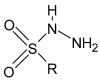
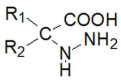
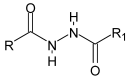
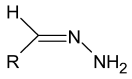
Schiff bases are a vast group of compounds characterized by the presence of a double bond linking carbon and nitrogen atoms, the versatility of which is generated in the many ways to combine a variety of alkyl or aryl substituents. Compounds of this type are both found in nature and synthesized in the laboratory. For years, Schiff bases have been greatly inspiring to many chemists and biochemists. In this article, we attempt to present a new take on this group of compounds, underlining of the importance of various types of Schiff bases. Among the different types of compounds that can be classified as Schiff bases, we chose hydrazides, dihydrazides, hydrazones and mixed derivatives such as hydrazide-hydrazones. For these compounds, we presented the elements of their structure that allow them to be classified as Schiff bases. While hydrazones are typical examples of Schiff bases, including hydrazides among them may be surprising for some. In their case, this is possible due to the amide-iminol tautomerism. The carbon-nitrogen double bond present in the iminol tautomer is a typical element found in Schiff bases. In addition to the characteristics of the structure of these selected derivatives, and sometimes their classification, we presented selected literature items which, in our opinion, represent their importance in various fields well.
Keywords: Schiff bases; dihydrazides; hydrazides; hydrazide–hydrazones; hydrazones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
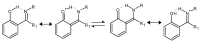


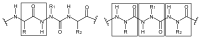
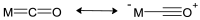


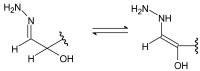
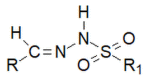


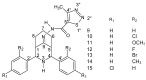
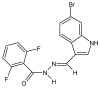
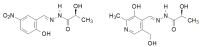
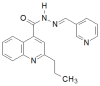
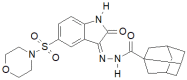
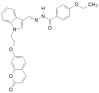
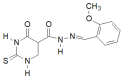
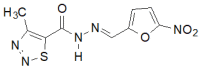
Similar articles
-
A Captivating Potential of Schiff Bases Derivatives for Antidiabetic Activity.Curr Pharm Des. 2025;31(1):37-56. doi: 10.2174/0113816128339161240913055034. Curr Pharm Des. 2025. PMID: 39313905 Review.
-
CP/MAS spectroscopy in the determination of the tautomeric forms of gossypol, its Schiff bases and hydrazones in the solid state.Magn Reson Chem. 2008 Jun;46(6):534-44. doi: 10.1002/mrc.2210. Magn Reson Chem. 2008. PMID: 18338327
-
Effective methods for the synthesis of hydrazones, quinazolines, and Schiff bases: reaction monitoring using a chemometric approach.RSC Adv. 2020 Oct 20;10(63):38566-38577. doi: 10.1039/d0ra06845d. eCollection 2020 Oct 15. RSC Adv. 2020. PMID: 35517547 Free PMC article.
-
Tautomerism and stereodynamics in Schiff bases from gossypol and hemigossypol with N-aminoheterocycles.Org Biomol Chem. 2019 Jun 26;17(25):6229-6250. doi: 10.1039/c9ob01011d. Org Biomol Chem. 2019. PMID: 31183482
-
Electrochemical Sensors Containing Schiff Bases and their Transition Metal Complexes to Detect Analytes of Forensic, Pharmaceutical and Environmental Interest. A Review.Crit Rev Anal Chem. 2019;49(6):488-509. doi: 10.1080/10408347.2018.1561242. Epub 2019 Feb 15. Crit Rev Anal Chem. 2019. PMID: 30767567 Review.
Cited by
-
Dynamic Covalent Bond-Based Polymer Chains Operating Reversibly with Temperature Changes.Molecules. 2024 Jul 10;29(14):3261. doi: 10.3390/molecules29143261. Molecules. 2024. PMID: 39064840 Free PMC article. Review.
-
Investigation of a novel Schiff base Catena-((μ6-(E)-2-((4-methoxy-2-oxidobenzylidene)ammonio)ethane-1-sulfonato potassium as a potential antibacterial agent.J Mol Model. 2024 Dec 19;31(1):25. doi: 10.1007/s00894-024-06219-1. J Mol Model. 2024. PMID: 39699755
-
Antioxidant and Anticancer Potential of the New Cu(II) Complexes Bearing Imine-Phenolate Ligands with Pendant Amine N-Donor Groups.Pharmaceutics. 2023 Jan 22;15(2):376. doi: 10.3390/pharmaceutics15020376. Pharmaceutics. 2023. PMID: 36839698 Free PMC article.
-
Promising Schiff bases in antiviral drug design and discovery.Med Chem Res. 2023;32(6):1063-1076. doi: 10.1007/s00044-023-03068-0. Epub 2023 May 10. Med Chem Res. 2023. PMID: 37305208 Free PMC article. Review.
-
Discovery of new Schiff bases of the disalicylic acid scaffold as DNA gyrase and topoisomerase IV inhibitors endowed with antibacterial properties.Front Chem. 2024 Jun 7;12:1419242. doi: 10.3389/fchem.2024.1419242. eCollection 2024. Front Chem. 2024. PMID: 38911996 Free PMC article.
References
-
- Schiff H. Mittheilungen aus dem Universitäts-laboratorium in Pisa: 2. Eine neue Reihe organischer Basen [Communications from the university laboratory in Pisa: 2. A new series of organic bases] Ann. Der Chem. Und Pharm. 1864;131:118–119. doi: 10.1002/jlac.18641310113. (In German) - DOI
-
- Moss G.P., Smith P.A.S., Tavernier D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995) Pure Appl. Chem. 1995;67:1307–1375. doi: 10.1351/pac199567081307. - DOI
-
- Pfeiffer P., Breith E., Llibbe E., Tsumaki T. Tricyclische orthokondensierte Nebenvalenzringe. Justus Liebigs Ann. Chem. 1933;503:84–130. doi: 10.1002/jlac.19335030106. - DOI
-
- Hunter L., Marriott J.A. Co-ordinated copper and nickel compounds of salicylidene derivatives. J. Chem. Soc. 1937;422:2000–2003. doi: 10.1039/jr9370002000. - DOI
-
- Sacconi L., Ciampolini M., Maggio F., Cavasini F.P. Studies in Coordination Chemistry. IX.1Investigation of the Stereochemistry of Some Complex Compounds of Cobalt(II) with N-Substituted Salicylaldimines. J. Am. Chem. Soc. 1962;84:3246–3248. doi: 10.1021/ja00876a005. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

