Exploring Intra- and Intermolecular Interactions in Selected N-Oxides-The Role of Hydrogen Bonds
- PMID: 35164056
- PMCID: PMC8846293
- DOI: 10.3390/molecules27030792
Exploring Intra- and Intermolecular Interactions in Selected N-Oxides-The Role of Hydrogen Bonds
Abstract
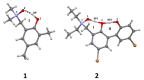
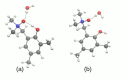
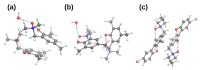
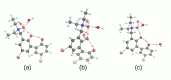
Intra- and intermolecular interactions have been explored in selected N-oxide derivatives: 2-(N,N-dimethylamino-N-oxymethyl)-4,6-dimethylphenyl (1) and 5,5'-dibromo-3-diethylaminomethyl-2,2'-biphenol N-oxide (2). Both compounds possess intramolecular hydrogen bonding, which is classified as moderate in 1 and strong in 2, and resonance-assisted in both cases. Density Functional Theory (DFT) in its classical formulation as well as Time-Dependent extension (TD-DFT) were employed to study proton transfer phenomena. The simulations were performed in the gas phase and with implicit and explicit solvation models. The obtained structures of the studied N-oxides were compared with experimental data available. The proton reaction path was investigated using scan with an optimization method, and water molecule reorientation in the monohydrate of 1 was found upon the proton scan progress. It was found that spontaneous proton transfer phenomenon cannot occur in the electronic ground state of the compound 1. An opposite situation was noticed for the compound 2. The changes of nucleophilicity and electrophilicity upon the bridged proton migration were analyzed on the basis of Fukui functions in the case of 1. The interaction energy decomposition of dimers and microsolvation models was investigated using Symmetry-Adapted Perturbation Theory (SAPT). The simulations were performed in both phases to introduce polar environment influence on the interaction energies. The SAPT study showed rather minor role of induction in the formation of homodimers. However, it is worth noticing that the same induction term is responsible for the preference of water molecules' interaction with N-oxide hydrogen bond acceptor atoms in the microsolvation study. The Natural Bond Orbital (NBO) analysis was performed for the complexes with water to investigate the charge flow upon the polar environment introduction. Finally, the TD-DFT was applied for isolated molecules as well as for microsolvation models showing that the presence of solvent affects excited states, especially when the N-oxide acceptor atom is microsolvated.
Keywords: DFT; Fukui functions; IEF-PCM; N-oxides; NBO; SAPT; TD-DFT; microsolvation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Revealing Intra- and Intermolecular Interactions Determining Physico-Chemical Features of Selected Quinolone Carboxylic Acid Derivatives.Molecules. 2022 Apr 1;27(7):2299. doi: 10.3390/molecules27072299. Molecules. 2022. PMID: 35408698 Free PMC article.
-
Competition of Intra- and Intermolecular Forces in Anthraquinone and Its Selected Derivatives.Molecules. 2021 Jun 6;26(11):3448. doi: 10.3390/molecules26113448. Molecules. 2021. PMID: 34204133 Free PMC article.
-
Naphthazarin Derivatives in the Light of Intra- and Intermolecular Forces.Molecules. 2021 Sep 17;26(18):5642. doi: 10.3390/molecules26185642. Molecules. 2021. PMID: 34577113 Free PMC article.
-
Non-Covalent Forces in Naphthazarin-Cooperativity or Competition in the Light of Theoretical Approaches.Int J Mol Sci. 2021 Jul 27;22(15):8033. doi: 10.3390/ijms22158033. Int J Mol Sci. 2021. PMID: 34360798 Free PMC article.
-
Hydrogen bond strengthening between o-nitroaniline and formaldehyde in electronic excited states: A theoretical study.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Jun 15;199:194-201. doi: 10.1016/j.saa.2018.03.062. Epub 2018 Mar 24. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29605783 Review.
Cited by
-
Synthesis, crystal structure, and a molecular modeling approach to identify effective antiviral hydrazide derivative against the main protease of SARS-CoV-2.J Mol Struct. 2022 Oct 5;1265:133391. doi: 10.1016/j.molstruc.2022.133391. Epub 2022 May 28. J Mol Struct. 2022. PMID: 35663190 Free PMC article.
-
Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and Computational Approach of a New Pyrazolo[3,4-g]isoquinoline Derivative as Potent against Leucine-Rich Repeat Kinase 2 (LRRK2).ACS Omega. 2024 Jul 1;9(28):30751-30770. doi: 10.1021/acsomega.4c03208. eCollection 2024 Jul 16. ACS Omega. 2024. PMID: 39035914 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

