Part I: NiMoO4 Nanostructures Synthesized by the Solution Combustion Method: A Parametric Study on the Influence of Synthesis Parameters on the Materials' Physicochemical, Structural, and Morphological Properties
- PMID: 35164057
- PMCID: PMC8839866
- DOI: 10.3390/molecules27030776
Part I: NiMoO4 Nanostructures Synthesized by the Solution Combustion Method: A Parametric Study on the Influence of Synthesis Parameters on the Materials' Physicochemical, Structural, and Morphological Properties
Abstract
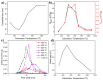
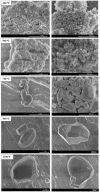
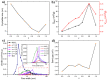
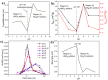
The impact of process conditions on the synthesis of NiMoO4 nanostructures using a solution combustion synthesis (SCS) method, in which agar powder and Ni(NO3)2 were utilized as fuel and as the oxidant, respectively, was thoroughly studied. The results show that the calcination temperature had a significant implication on the specific surface area, phase composition, particle size, band gap, and crystallite size. The influence of calcination time on the resulting physicochemical/structural/morphological properties of NiMoO4 nanostructures was found to be a major effect during the first 20 min, beyond which these properties varied to a lesser extent. The increase in the Ni/Mo atomic ratio in the oxide impacted the combustion dynamics of the system, which led to the formation of higher surface area materials, with the prevalence of the β-phase in Ni-rich samples. Likewise, the change in the pH of the precursor solution showed that the combustion reaction is more intense in the high-pH region, entailing major implications on the physicochemical properties and phase composition of the samples. The change in the fuel content showed that the presence of agar is important, as it endows the sample with a fluffy, porous texture and is also vital for the preponderance of the β-phase.
Keywords: agar; nanostructures; nickel molybdate; parametric study; physicochemical properties; solution combustion synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Part II: NiMoO4 Nanostructures Synthesized by the Solution Combustion Method: A Parametric Study on the Influence of Material Synthesis and Electrode-Fabrication Parameters on the Electrocatalytic Activity in the Hydrogen Evolution Reaction.Molecules. 2022 Feb 10;27(4):1199. doi: 10.3390/molecules27041199. Molecules. 2022. PMID: 35208991 Free PMC article.
-
Solution Combustion Synthesis of Ni-Based Nanocatalyst Using Ethylenediaminetetraacetic Acid and Nickel-Carbon Nanotube Growth Behavior.Materials (Basel). 2023 Nov 16;16(22):7191. doi: 10.3390/ma16227191. Materials (Basel). 2023. PMID: 38005120 Free PMC article.
-
Synthesis and structural characterization of Mo-Ni-W oxide nanostructures.J Nanosci Nanotechnol. 2008 Jun;8(6):2983-9. doi: 10.1166/jnn.2008.117. J Nanosci Nanotechnol. 2008. PMID: 18681035
-
Solution Combustion Synthesis: Towards a Sustainable Approach for Metal Oxides.Chemistry. 2020 Jul 27;26(42):9099-9125. doi: 10.1002/chem.202000678. Epub 2020 Apr 21. Chemistry. 2020. PMID: 32134133 Review.
-
Insight into nanocrystal synthesis: from precursor decomposition to combustion.RSC Adv. 2022 Aug 30;12(37):24374-24389. doi: 10.1039/d2ra05222a. eCollection 2022 Aug 22. RSC Adv. 2022. PMID: 36128523 Free PMC article. Review.
Cited by
-
Part II: NiMoO4 Nanostructures Synthesized by the Solution Combustion Method: A Parametric Study on the Influence of Material Synthesis and Electrode-Fabrication Parameters on the Electrocatalytic Activity in the Hydrogen Evolution Reaction.Molecules. 2022 Feb 10;27(4):1199. doi: 10.3390/molecules27041199. Molecules. 2022. PMID: 35208991 Free PMC article.
References
-
- Gawande M.B., Pandey R.K., Jayaram R.V. Role of mixed metal oxides in catalysis science—Versatile applications in organic synthesis. Catal. Sci. 2012;2:1113–1125. doi: 10.1039/c2cy00490a. - DOI
-
- Madeira L., Portela M., Mazzocchia C. Nickel molybdate catalysts and their use in the selective oxidation of hydrocarbons. Catal. Rev. 2004;46:53–110. doi: 10.1081/CR-120030053. - DOI
-
- Jothi P.R., Shanthi K., Salunkhe R.R., Pramanik M., Malgras V., Alshehri S.M., Yamauchi Y. Synthesis and Characterization of α-NiMoO4 Nanorods for Supercapacitor Application. Eur. J. Inorg. Chem. 2015;2015:3694–3699. doi: 10.1002/ejic.201500410. - DOI
-
- Adhikary M.C., Priyadarsini M., Rath S.K., Das C.K. 3D porous NiMoO4 nanoflakes arrays for advanced supercapacitor electrodes. J. Nanopart. Res. 2017;19:314. doi: 10.1007/s11051-017-4008-2. - DOI
-
- Yesuraj J., Padmaraj O., Suthanthiraraj S.A. Synthesis, Characterization, and Improvement of Supercapacitor Properties of NiMoO4 Nanocrystals with Polyaniline. J. Inorg. Organomet. Polym. 2020;30:310–321. doi: 10.1007/s10904-019-01189-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

