2-Hydroxyestradiol Overcomes Mesenchymal Stem Cells-Mediated Platinum Chemoresistance in Ovarian Cancer Cells in an ERK-Independent Fashion
- PMID: 35164068
- PMCID: PMC8839885
- DOI: 10.3390/molecules27030804
2-Hydroxyestradiol Overcomes Mesenchymal Stem Cells-Mediated Platinum Chemoresistance in Ovarian Cancer Cells in an ERK-Independent Fashion
Abstract
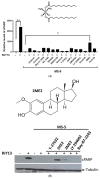
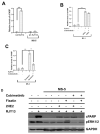
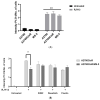
Ovarian cancer (OC) is the second most common type of gynecological malignancy. Platinum (Pt)-based chemotherapy is the standard of care for OC, but toxicity and acquired chemoresistance has proven challenging. Recently, we reported that sensitivity to platinum was significantly reduced in a co-culture of OC cells with MSC. To discover compounds capable of restoring platinum sensitivity, we screened a number of candidates and monitored ability to induce PARP cleavage. Moreover, we monitored platinum uptake and expression of ABC transporters in OC cells. Our results showed that 2-hydroxyestradiol (2HE2), a metabolite of estradiol, and dasatinib, an Abl/Src kinase inhibitor, were significantly effective in overcoming MSC-mediated platinum drug resistance. Dasatinib activity was dependent on ERK1/2 activation, whereas 2HE2 was independent of the activation of ERK1/2. MSC-mediated platinum drug resistance was accompanied by reduced intracellular platinum concentrations in OC cells. Moreover, MSC co-cultured with OC cells resulted in downregulation of the expression of cellular transporters required for platinum uptake and efflux. Exposure to 2HE2 and other modulators resulted in an increase in intracellular platinum concentrations. Thus, 2HE2 and dasatinib might act as sensitizers to restore platinum drug sensitivity to OC cells and thus to limit TME-mediated chemoresistance in OC.
Keywords: 2HE2; ERK; drug resistance; ovarian cancer; platinum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Secreted Soluble Factors from Tumor-Activated Mesenchymal Stromal Cells Confer Platinum Chemoresistance to Ovarian Cancer Cells.Int J Mol Sci. 2023 Apr 23;24(9):7730. doi: 10.3390/ijms24097730. Int J Mol Sci. 2023. PMID: 37175439 Free PMC article.
-
Flavonoids Restore Platinum Drug Sensitivity to Ovarian Carcinoma Cells in a Phospho-ERK1/2-Dependent Fashion.Int J Mol Sci. 2020 Sep 7;21(18):6533. doi: 10.3390/ijms21186533. Int J Mol Sci. 2020. PMID: 32906729 Free PMC article.
-
Ovarian cancer ascites confers platinum chemoresistance to ovarian cancer cells.Transl Oncol. 2024 Jun;44:101939. doi: 10.1016/j.tranon.2024.101939. Epub 2024 Mar 14. Transl Oncol. 2024. PMID: 38489872 Free PMC article.
-
Mechanisms underlying acquired platinum resistance in high grade serous ovarian cancer - a mini review.Biochim Biophys Acta Gen Subj. 2019 Feb;1863(2):371-378. doi: 10.1016/j.bbagen.2018.11.005. Epub 2018 Nov 10. Biochim Biophys Acta Gen Subj. 2019. PMID: 30423357 Review.
-
Tyrosine-kinases inhibitors in recurrent platinum-resistant ovarian cancer patients.Cancer Treat Rev. 2016 Jan;42:41-6. doi: 10.1016/j.ctrv.2015.10.011. Epub 2015 Nov 10. Cancer Treat Rev. 2016. PMID: 26559739 Review.
Cited by
-
Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice.Foods. 2024 Nov 30;13(23):3881. doi: 10.3390/foods13233881. Foods. 2024. PMID: 39682952 Free PMC article.
-
Secreted Soluble Factors from Tumor-Activated Mesenchymal Stromal Cells Confer Platinum Chemoresistance to Ovarian Cancer Cells.Int J Mol Sci. 2023 Apr 23;24(9):7730. doi: 10.3390/ijms24097730. Int J Mol Sci. 2023. PMID: 37175439 Free PMC article.
References
-
- Cohen S.M., Lippard S.J. Cisplatin: From DNA damage to cancer chemotherapy. Prog. Nucleic Acid Res. Mol. Biol. 2001;67:93–130. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

