Spin Trapping Hydroxyl and Aryl Radicals of One-Electron Reduced Anticancer Benzotriazine 1,4-Dioxides
- PMID: 35164077
- PMCID: PMC8840461
- DOI: 10.3390/molecules27030812
Spin Trapping Hydroxyl and Aryl Radicals of One-Electron Reduced Anticancer Benzotriazine 1,4-Dioxides
Abstract
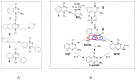
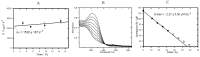
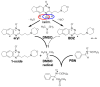
Hypoxia in tumors results in resistance to both chemotherapy and radiotherapy treatments but affords an environment in which hypoxia-activated prodrugs (HAP) are activated upon bioreduction to release targeted cytotoxins. The benzotriazine 1,4-di-N-oxide (BTO) HAP, tirapazamine (TPZ, 1), has undergone extensive clinical evaluation in combination with radiotherapy to assist in the killing of hypoxic tumor cells. Although compound 1 did not gain approval for clinical use, it has spurred on the development of other BTOs, such as the 3-alkyl analogue, SN30000, 2. There is general agreement that the cytotoxin(s) from BTOs arise from the one-electron reduced form of the compounds. Identifying the cytotoxic radicals, and whether they play a role in the selective killing of hypoxic tumor cells, is important for continued development of the BTO class of anticancer prodrugs. In this study, nitrone spin-traps, combined with electron spin resonance, give evidence for the formation of aryl radicals from compounds 1, 2 and 3-phenyl analogues, compounds 3 and 4, which form carbon C-centered radicals. In addition, high concentrations of DEPMPO (5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide) spin-trap the •OH radical. The combination of spin-traps with high concentrations of DMSO and methanol also give evidence for the involvement of strongly oxidizing radicals. The failure to spin-trap methyl radicals with PBN (N-tert-butylphenylnitrone) on the bioreduction of compound 2, in the presence of DMSO, implies that free •OH radicals are not released from the protonated radical anions of compound 2. The spin-trapping of •OH radicals by high concentrations of DEPMPO, and the radical species arising from DMSO and methanol give both direct and indirect evidence for the scavenging of •OH radicals that are involved in an intramolecular process. Hypoxia-selective cytotoxicity is not related to the formation of aryl radicals from the BTO compounds as they are associated with high aerobic cytotoxicity.
Keywords: aryl radical; benzotriazine 1,4-dioxide; cytochrome P450 oxidoreductase; cytotoxicity; electron spin resonance; hydroxyl radical; hypoxia-activated prodrug; tirapazamine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


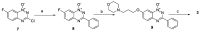
References
-
- Baker M.A., Zeman E.M., Hirst V.K., Brown J.M. Metabolism of SR 4233 by chinese hamster ovary cells: Basis of selective hypoxic cytotoxicity. Cancer Res. 1988;48:5947–5952. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

