Anti-Osteoporosis Effect of Perilla frutescens Leaf Hexane Fraction through Regulating Osteoclast and Osteoblast Differentiation
- PMID: 35164085
- PMCID: PMC8840259
- DOI: 10.3390/molecules27030824
Anti-Osteoporosis Effect of Perilla frutescens Leaf Hexane Fraction through Regulating Osteoclast and Osteoblast Differentiation
Abstract
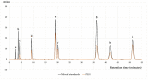
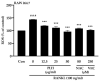
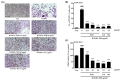
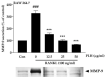
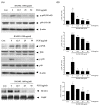
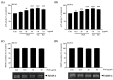
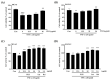
Osteoporosis is the result of an imbalance in the bone-remodeling process via an increase in osteoclastic activity and a decrease in osteoblastic activity. Our previous studies have shown that Perilla frutescens seed meal has anti-osteoclastogenic activity. However, the role of perilla leaf hexane fraction (PLH) in osteoporosis has not yet been investigated and reported. In this study, we aimed to investigate the effects of PLH in osteoclast differentiation and osteogenic potential using cell-based experiments in vitro. From HPLC analysis, we found that PLH contained high luteolin and baicalein. PLH was shown to inhibit RANKL-induced ROS production and tartrate-resistant acid phosphatase (TRAP)-positive multi-nucleated osteoclasts. Moreover, PLH significantly downregulated the RANKL-induced MAPK and NF-κB signaling pathways, leading to the attenuation of NFATc1 and MMP-9 expression. In contrast, PLH enhanced osteoblast function by regulating alkaline phosphatase (ALP) and restoring TNF-α-suppressed osteoblast proliferation and osteogenic potential. Thus, luteolin and baicalein-rich PLH inhibits osteoclast differentiation but promotes the function of osteoblasts. Collectively, our data provide new evidence that suggests that PLH may be a valuable anti-osteoporosis agent.
Keywords: Perilla frutescens; RANKL; TNF-α; osteoblast; osteoclast; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Leonurus sibiricus L. ethanol extract promotes osteoblast differentiation and inhibits osteoclast formation.Int J Mol Med. 2019 Sep;44(3):913-926. doi: 10.3892/ijmm.2019.4269. Epub 2019 Jul 8. Int J Mol Med. 2019. PMID: 31524244 Free PMC article.
-
Macrolactin A protects against LPS-induced bone loss by regulation of bone remodeling.Eur J Pharmacol. 2020 Sep 15;883:173305. doi: 10.1016/j.ejphar.2020.173305. Epub 2020 Jul 14. Eur J Pharmacol. 2020. PMID: 32673673
-
Chaenomelis fructus inhibits osteoclast differentiation by suppressing NFATc1 expression and prevents ovariectomy-induced osteoporosis.BMC Complement Med Ther. 2020 Feb 5;20(1):35. doi: 10.1186/s12906-020-2841-9. BMC Complement Med Ther. 2020. PMID: 32024503 Free PMC article.
-
Water extract of Rumex crispus prevents bone loss by inhibiting osteoclastogenesis and inducing osteoblast mineralization.BMC Complement Altern Med. 2017 Oct 26;17(1):483. doi: 10.1186/s12906-017-1986-7. BMC Complement Altern Med. 2017. PMID: 29070038 Free PMC article.
-
The Role and Mechanism of Perilla frutescens in Cancer Treatment.Molecules. 2023 Aug 4;28(15):5883. doi: 10.3390/molecules28155883. Molecules. 2023. PMID: 37570851 Free PMC article. Review.
Cited by
-
Anthocyanins from a new hybrid sweet potato peel cultivated in Northern Thailand mitigate LPS-induced inflammation and RANKL-induced osteoporosis by regulating ROS-mediated pathways.Inflammopharmacology. 2025 Jan;33(1):381-399. doi: 10.1007/s10787-024-01634-5. Epub 2025 Jan 13. Inflammopharmacology. 2025. PMID: 39806052 Free PMC article.
-
Targeting lipid raft-related stomatin to ameliorate osteoporosis in preclinical models.Nat Commun. 2025 Jul 1;16(1):5495. doi: 10.1038/s41467-025-60032-9. Nat Commun. 2025. PMID: 40595453 Free PMC article.
-
The role of lipid metabolism in osteoporosis: Clinical implication and cellular mechanism.Genes Dis. 2023 Sep 20;11(4):101122. doi: 10.1016/j.gendis.2023.101122. eCollection 2024 Jul. Genes Dis. 2023. PMID: 38523674 Free PMC article. Review.
-
A Review on the Molecular Mechanisms of Action of Natural Products in Preventing Bone Diseases.Int J Mol Sci. 2022 Jul 30;23(15):8468. doi: 10.3390/ijms23158468. Int J Mol Sci. 2022. PMID: 35955603 Free PMC article. Review.
-
Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID.Front Med (Lausanne). 2023 Jan 9;9:1072056. doi: 10.3389/fmed.2022.1072056. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36698809 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

