Structural Insight and Development of EGFR Tyrosine Kinase Inhibitors
- PMID: 35164092
- PMCID: PMC8838133
- DOI: 10.3390/molecules27030819
Structural Insight and Development of EGFR Tyrosine Kinase Inhibitors
Abstract
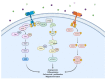
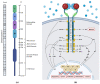
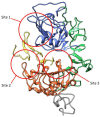
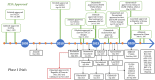
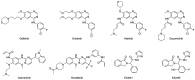
Lung cancer has a high prevalence, with a growing number of new cases and mortality every year. Furthermore, the survival rate of patients with non-small-cell lung carcinoma (NSCLC) is still quite low in the majority of cases. Despite the use of conventional therapy such as tyrosine kinase inhibitor for Epidermal Growth Factor Receptor (EGFR), which is highly expressed in most NSCLC cases, there was still no substantial improvement in patient survival. This is due to the drug's ineffectiveness and high rate of resistance among individuals with mutant EGFR. Therefore, the development of new inhibitors is urgently needed. Understanding the EGFR structure, including its kinase domain and other parts of the protein, and its activation mechanism can accelerate the discovery of novel compounds targeting this protein. This study described the structure of the extracellular, transmembrane, and intracellular domains of EGFR. This was carried out along with identifying the binding pose of commercially available inhibitors in the ATP-binding and allosteric sites, thereby clarifying the research gaps that can be filled. The binding mechanism of inhibitors that have been used clinically was also explained, thereby aiding the structure-based development of new drugs.
Keywords: EGFR; activation; binding; inhibitor; kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

