Iboga Inspired N-Indolylethyl-Substituted Isoquinuclidines as a Bioactive Scaffold: Chemoenzymatic Synthesis and Characterization as GDNF Releasers and Antitrypanosoma Agents
- PMID: 35164094
- PMCID: PMC8839081
- DOI: 10.3390/molecules27030829
Iboga Inspired N-Indolylethyl-Substituted Isoquinuclidines as a Bioactive Scaffold: Chemoenzymatic Synthesis and Characterization as GDNF Releasers and Antitrypanosoma Agents
Abstract
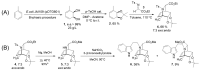
The first stage of the drug discovery process involves the identification of small compounds with biological activity. Iboga alkaloids are monoterpene indole alkaloids (MIAs) containing a fused isoquinuclidine-tetrahydroazepine ring. Both the natural products and the iboga-inspired synthetic analogs have shown a wide variety of biological activities. Herein, we describe the chemoenzymatic preparation of a small library of novel N-indolylethyl-substituted isoquinuclidines as iboga-inspired compounds, using toluene as a starting material and an imine Diels-Alder reaction as the key step in the synthesis. The new iboga series was investigated for its potential to promote the release of glial cell line-derived neurotrophic factor (GDNF) by C6 glioma cells, and to inhibit the growth of infective trypanosomes. GDNF is a neurotrophic factor widely recognized by its crucial role in development, survival, maintenance, and protection of dopaminergic neuronal circuitries affected in several neurological and psychiatric pathologies. Four compounds of the series showed promising activity as GDNF releasers, and a leading structure (compound 11) was identified for further studies. The same four compounds impaired the growth of bloodstream Trypanosoma brucei brucei (EC50 1-8 μM) and two of them (compounds 6 and 14) showed a good selectivity index.
Keywords: GDNF; anti-trypanosoma; iboga alkaloid; imino Diels–Alder; isoquinuclidine; toluenedioxigenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


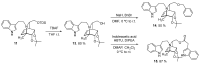

References
-
- Marton S., González B., Rodríguez-Bottero S., Miquel E., Martínez-Palma L., Pazos M., Prieto J.P., Rodríguez P., Sames D., Seoane G., et al. Ibogaine administration modifies GDNF and BDNF expression in brain regions involved in mesocorticolimbic and nigral dopaminergic circuits. Front. Pharmacol. 2019;10:193. doi: 10.3389/fphar.2019.00193. - DOI - PMC - PubMed
-
- He D.-Y., Mcgough N.N.H., Ravindranathan A., Jeanblanc J., Logrip M.L., Phamluong K., Janak P.H., Ron D. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J. Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

