Experimental Investigation on Thermophysical Properties of Ammonium-Based Protic Ionic Liquids and Their Potential Ability towards CO2 Capture
- PMID: 35164113
- PMCID: PMC8839255
- DOI: 10.3390/molecules27030851
Experimental Investigation on Thermophysical Properties of Ammonium-Based Protic Ionic Liquids and Their Potential Ability towards CO2 Capture
Abstract
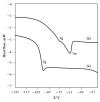
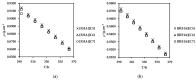
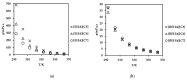
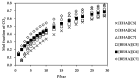
Ionic liquids, which are extensively known as low-melting-point salts, have received significant attention as the promising solvent for CO2 capture. This work presents the synthesis, thermophysical properties and the CO2 absorption of a series of ammonium cations coupled with carboxylate anions producing ammonium-based protic ionic liquids (PILs), namely 2-ethylhexylammonium pentanoate ([EHA][C5]), 2-ethylhexylammonium hexanoate ([EHA][C6]), 2-ethylhexylammonium heptanoate ([EHA][C7]), bis-(2-ethylhexyl)ammonium pentanoate ([BEHA][C5]), bis-(2-ethylhexyl)ammonium hexanoate ([BEHA][C6]) and bis-(2-ethylhexyl)ammonium heptanoate ([BEHA][C7]). The chemical structures of the PILs were confirmed by using Nuclear Magnetic Resonance (NMR) spectroscopy while the density (ρ) and the dynamic viscosity (η) of the PILs were determined and analyzed in a range from 293.15K up to 363.15K. The refractive index (nD) was also measured at T = (293.15 to 333.15) K. Thermal analyses conducted via a thermogravimetric analyzer (TGA) and differential scanning calorimeter (DSC) indicated that all PILs have the thermal decomposition temperature, Td of greater than 416K and the presence of glass transition, Tg was detected in each PIL. The CO2 absorption of the PILs was studied up to 29 bar at 298.15 K and the experimental results showed that [BEHA][C7] had the highest CO2 absorption with 0.78 mol at 29 bar. The CO2 absorption values increase in the order of [C5] < [C6] < [C7] anion regardless of the nature of the cation.
Keywords: CO2 absorption; ammonium-based protic ionic liquids; density; lattice potential energy; phase transition; refractive index; standard entropy; thermal expansion coefficient; viscosity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Muda N., Jin T. On prediction of depreciation time of fossil fuel in Malaysia. J. Math Stat. 2012;8:136–143. doi: 10.3844/jmssp.2012.136.143. - DOI
-
- Mokhatab S., Poe W.A., Mak J.Y., editors. Handbook of Natural Gas Transmission and Processing. 3rd ed. Gulf Professional Publishing; Boston, MA, USA: 2015. Chapter 1—Natural Gas Fundamentals; pp. 1–36.
-
- Guo B., Ghalambor A., editors. Natural Gas Engineering Handbook. 2nd ed. Gulf Publishing Company; Houston, TX, USA: 2005. Chapter 1—Introduction; pp. 1–11.
-
- Speight J.G., editor. Natural Gas. 2nd ed. Gulf Professional Publishing; Boston, MA, USA: 2019. 5—Recovery, Storage, and Transportation; pp. 149–186.
-
- Liang Z., Rongwong W., Liu H., Fu K., Gao H., Cao F., Zhang R., Sema T., Henni A., Sumon K., et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control. 2015;40:26–54. doi: 10.1016/j.ijggc.2015.06.017. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

