Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient
- PMID: 35164119
- PMCID: PMC8838834
- DOI: 10.3390/molecules27030861
Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient
Abstract
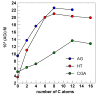
Selecting effective antioxidants is challenging since their efficiency in inhibiting lipid oxidation depends on the rate constants of the chemical reactions involved and their concentration at the reaction site, i.e., at the interfacial region. Accumulation of antioxidants at the interface of emulsions is key to modulate their efficiency in inhibiting lipid oxidation but its control was not well understood, especially in emulsions. It can be optimized by modifying the physicochemical properties of antioxidants or the environmental conditions. In this work, we analyze the effects of surfactant concentration, droplet size, and oil to water ratio on the effective interfacial concentration of a set of chlorogenic acid (CGA) esters in fish oil-in-water (O/W) emulsions and nanoemulsions and on their antioxidant efficiency. A well-established pseudophase kinetic model is used to determine in the intact emulsified systems the effective concentrations of the antioxidants (AOs). The relative oxidative stability of the emulsions is assessed by monitoring the formation of primary oxidation products with time. Results show that the concentration of all AOs at the interfacial region is much higher (20-90 fold) than the stoichiometric one but is much lower than those of other phenolipid series such as caffeic or hydroxytyrosol derivatives. The main parameter controlling the interfacial concentration of antioxidants is the surfactant volume fraction, ΦI, followed by the O/W ratio. Changes in the droplet sizes (emulsions and nanoemulsions) have no influence on the interfacial concentrations. Despite the high radical scavenging capacity of CGA derivatives and their being concentrated at the interfacial region, the investigated AOs do not show a significant effect in inhibiting lipid oxidation in contrast with what is observed using other series of homologous antioxidants with similar reactivity. Results are tentatively interpreted in terms of the relatively low interfacial concentrations of the antioxidants, which may not be high enough to make the rate of the inhibition reaction faster than the rate of radical propagation.
Keywords: antioxidants; chlorogenic acid; distribution; droplet size; emulsion; interfacial concentration; lipid oxidation; nanoemulsion; phenolipids; pseudophase kinetic model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
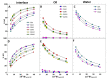
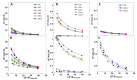
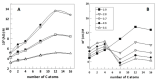
 —ΦI = 0.005,
—ΦI = 0.005,  —ΦI = 0.01,
—ΦI = 0.01,  —ΦI = 0.02) at different emulsifier volume fractions. (B) Effect of O/W ratio on interfacial AOs concentration in nanoemulsions at ΦI = 0.005. In both graphs, [AOT] = 0.125 mM.
—ΦI = 0.02) at different emulsifier volume fractions. (B) Effect of O/W ratio on interfacial AOs concentration in nanoemulsions at ΦI = 0.005. In both graphs, [AOT] = 0.125 mM.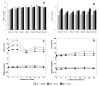
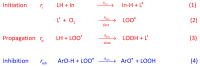
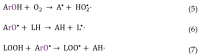
Similar articles
-
Interfacial Concentrations of Hydroxytyrosol Derivatives in Fish Oil-in-Water Emulsions and Nanoemulsions and Its Influence on Their Lipid Oxidation: Droplet Size Effects.Foods. 2020 Dec 18;9(12):1897. doi: 10.3390/foods9121897. Foods. 2020. PMID: 33353202 Free PMC article.
-
Enhancement of the antioxidant efficiency of gallic acid derivatives in intact fish oil-in-water emulsions through optimization of their interfacial concentrations.Food Funct. 2018 Aug 15;9(8):4429-4442. doi: 10.1039/c8fo00977e. Food Funct. 2018. PMID: 30070303
-
Control of antioxidant efficiency of chlorogenates in emulsions: modulation of antioxidant interfacial concentrations.J Sci Food Agric. 2019 Jun;99(8):3917-3925. doi: 10.1002/jsfa.9615. Epub 2019 Mar 1. J Sci Food Agric. 2019. PMID: 30697750
-
Advances in the control of lipid peroxidation in oil-in-water emulsions: kinetic approaches†.Crit Rev Food Sci Nutr. 2023;63(23):6252-6284. doi: 10.1080/10408398.2022.2029827. Epub 2022 Feb 1. Crit Rev Food Sci Nutr. 2023. PMID: 35104177 Review.
-
What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox.Crit Rev Food Sci Nutr. 2015;55(2):183-201. doi: 10.1080/10408398.2011.650335. Crit Rev Food Sci Nutr. 2015. PMID: 24915410 Review.
Cited by
-
Analysis of the Efficiency of Antioxidants in Inhibiting Lipid Oxidation in Terms of Characteristic Kinetic Parameters.Antioxidants (Basel). 2024 May 11;13(5):593. doi: 10.3390/antiox13050593. Antioxidants (Basel). 2024. PMID: 38790698 Free PMC article.
-
Efficiency of δ-Tocopherol in Inhibiting Lipid Oxidation in Emulsions: Effects of Surfactant Charge and of Surfactant Concentration.Antioxidants (Basel). 2023 May 26;12(6):1158. doi: 10.3390/antiox12061158. Antioxidants (Basel). 2023. PMID: 37371888 Free PMC article.
References
-
- Jacobsen C., Nielsen N.S., Horn A.F., Sørensen A.-D.M. Food Enrichment with Omega-3 Fatty Acids. Elsevier; Amsterdam, The Netherlands: 2013.
-
- Arab-Tehrany E., Jacquot M., Gaiani C., Imran M., Desobry S., Linder M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012;25:24–33. doi: 10.1016/j.tifs.2011.12.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

