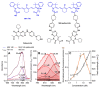
Conjugation of Palbociclib with MHI-148 Has an Increased Cytotoxic Effect for Breast Cancer Cells and an Altered Mechanism of Action
- PMID: 35164144
- PMCID: PMC8840619
- DOI: 10.3390/molecules27030880
Conjugation of Palbociclib with MHI-148 Has an Increased Cytotoxic Effect for Breast Cancer Cells and an Altered Mechanism of Action
Abstract
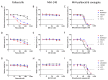
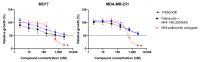
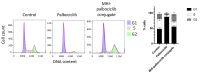
The CDK4/6 inhibitor palbociclib, combined with endocrine therapy, has been shown to be effective in postmenopausal women with estrogen receptor-positive, HER2-negative advanced or metastatic breast cancer. However, palbociclib is not as effective in the highly aggressive, triple-negative breast cancer that lacks sensitivity to chemotherapy or endocrine therapy. We hypothesized that conjugation of the near-infrared dye MHI-148 with palbociclib can produce a potential theranostic in triple-negative, as well as estrogen receptor-positive, breast cancer cells. In our study, the conjugate was found to have enhanced activity in all mammalian cell lines tested in vitro. However, the conjugate was cytotoxic and did not induce G1 cell cycle arrest in breast cancer cells, suggesting its mechanism of action differs from the parent compound palbociclib. The study highlights the importance of investigating the mechanism of conjugates of near-infrared dyes to therapeutic compounds, as conjugation can potentially result in a change of mechanism or target, with an enhanced cytotoxic effect in this case.
Keywords: CDK4/6 inhibitor palbociclib; MHI-148; breast cancer; cell cycle arrest; near-infrared fluorescent (NIRF).
Conflict of interest statement
All authors declare no conflict of interest.
Figures


References
-
- [(accessed on 23 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

