Healthy Properties of a New Formulation of Pomegranate-Peel Extract in Mice Suffering from Experimental Autoimmune Encephalomyelitis
- PMID: 35164175
- PMCID: PMC8838218
- DOI: 10.3390/molecules27030914
Healthy Properties of a New Formulation of Pomegranate-Peel Extract in Mice Suffering from Experimental Autoimmune Encephalomyelitis
Erratum in
-
Correction: Vallarino et al. Healthy Properties of a New Formulation of Pomegranate-Peel Extract in Mice Suffering from Experimental Autoimmune Encephalomyelitis. Molecules 2022, 27, 914.Molecules. 2025 Apr 15;30(8):1769. doi: 10.3390/molecules30081769. Molecules. 2025. PMID: 40333777 Free PMC article.
Abstract
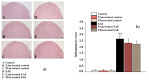
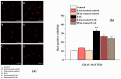
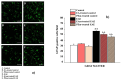
A new formulation of a pomegranate-peel extract (PEm) obtained by PUAE (Pulsed Ultrasound-Assisted Extraction) and titrated in both ellagic acid (EA) and punicalagin is proposed, characterized and then analyzed for potential health properties in mice suffering from the experimental autoimmune encephalomyelitis (EAE). PEm effects were compared to those elicited by a formulation containing EA (EAm). Control and EAE mice were chronically administered EAm and Pem dissolved in the drinking water, starting from the day 10 post-immunization (d.p.i.), with a "therapeutic" protocol to deliver daily 50 mg/kg of EA. Treated EAE mice did not limit their daily access to the beverage, nor did they show changes in body weight, but they displayed a significant amelioration of "in vivo" clinical symptoms. "Ex vivo" histochemical analysis showed that spinal-cord demyelination and inflammation in PEm and EAm-treated EAE mice at 23 ± 1 d.p.i. were comparable to those in the untreated EAE animals, while microglia activation (measured as Ionized Calcium Binding Adaptor 1, Iba1 staining) and astrocytosis (quantified as glial fibrillar acid protein, GFAP immunopositivity) significantly recovered, particularly in the gray matter. EAm and PEm displayed comparable efficiencies in controlling the spinal pathological cellular hallmarks in EAE mice, and this would support their delivery as dietary supplementation in patients suffering from multiple sclerosis (MS).
Keywords: astrocytosis; demyelination; ellagic acid; inflammation; multiple sclerosis; pomegranate peels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Parisio C., Lucarini E., Micheli L., Toti A., Khatib M., Mulinacci N., Calosi L., Bani D., Di Cesare Mannelli L., Ghelardini C. Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions. Int. J. Mol. Sci. 2020;21:4304. doi: 10.3390/ijms21124304. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

