Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process
- PMID: 35164219
- PMCID: PMC8839846
- DOI: 10.3390/molecules27030954
Effectively Converting Cane Molasses into 2,3-Butanediol Using Clostridiumljungdahlii by an Integrated Fermentation and Membrane Separation Process
Abstract
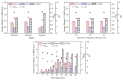
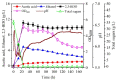
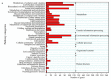
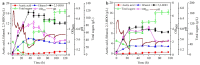
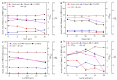
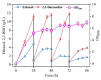
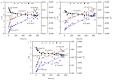
Firstly, 2,3-butanediol (2,3-BDO) is a chemical platform used in several applications. However, the pathogenic nature of its producers and the expensive feedstocks used limit its scale production. In this study, cane molasses was used for 2,3-BDO production by a nonpathogenic Clostridium ljungdahlii. It was found that cane molasses alone, without the addition of other ingredients, was favorable for use as the culture medium for 2,3-BDO production. Compared with the control (i.e., the modified DSMZ 879 medium), the differential genes are mainly involved in the pathways of carbohydrate metabolism, membrane transport, and amino acid metabolism in the case of the cane molasses alone. However, when cane molasses alone was used, cell growth was significantly inhibited by KCl in cane molasses. Similarly, a high concentration of sugars (i.e., above 35 g/L) can inhibit cell growth and 2,3-BDO production. More seriously, 2,3-BDO production was inhibited by itself. As a result, cane molasses alone with an initial 35 g/L total sugars was suitable for 2,3-BDO production in batch culture. Finally, an integrated fermentation and membrane separation process was developed to maintain high 2,3-BDO productivity of 0.46 g·L-1·h-1. Meanwhile, the varied fouling mechanism indicated that the fermentation properties changed significantly, especially for the cell properties. Therefore, the integrated fermentation and membrane separation process was favorable for 2,3-BDO production by C. ljungdahlii using cane molasses.
Keywords: 2,3-butanediol; Clostridium ljungdahlii; cane molasses; membrane separation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Techno-economic evaluation of a complete bioprocess for 2,3-butanediol production from renewable resources.Bioresour Technol. 2016 Mar;204:55-64. doi: 10.1016/j.biortech.2015.12.005. Epub 2015 Dec 17. Bioresour Technol. 2016. PMID: 26773945
-
Enhanced production of 2,3-butanediol from sugarcane molasses.Appl Biochem Biotechnol. 2015 Mar;175(6):3014-24. doi: 10.1007/s12010-015-1481-x. Epub 2015 Jan 14. Appl Biochem Biotechnol. 2015. PMID: 25586489
-
A newly isolated Enterobacter sp. strain produces 2,3-butanediol during its cultivation on low-cost carbohydrate-based substrates.FEMS Microbiol Lett. 2019 Jan 1;366(1). doi: 10.1093/femsle/fny280. FEMS Microbiol Lett. 2019. PMID: 30476146
-
Bioengineering for the industrial production of 2,3-butanediol by the yeast, Saccharomyces cerevisiae.World J Microbiol Biotechnol. 2022 Jan 12;38(3):38. doi: 10.1007/s11274-021-03224-x. World J Microbiol Biotechnol. 2022. PMID: 35018511 Review.
-
Metabolic engineering of non-pathogenic microorganisms for 2,3-butanediol production.Appl Microbiol Biotechnol. 2021 Aug;105(14-15):5751-5767. doi: 10.1007/s00253-021-11436-2. Epub 2021 Jul 21. Appl Microbiol Biotechnol. 2021. PMID: 34287658 Review.
Cited by
-
Food Waste from Campus Dining Hall as a Potential Feedstock for 2,3-Butanediol Production via Non-Sterilized Fermentation.Foods. 2024 Jan 31;13(3):452. doi: 10.3390/foods13030452. Foods. 2024. PMID: 38338586 Free PMC article.
-
Development of a Strategy for L-Lactic Acid Production by Rhizopus oryzae Using Zizania latifolia Waste and Cane Molasses as Carbon Sources.Molecules. 2023 Aug 24;28(17):6234. doi: 10.3390/molecules28176234. Molecules. 2023. PMID: 37687063 Free PMC article.
-
Highly efficient production of 2,3-butanediol from xylose and glucose by newly isolated thermotolerant Cronobacter sakazakii.BMC Microbiol. 2022 Jun 24;22(1):164. doi: 10.1186/s12866-022-02577-z. BMC Microbiol. 2022. PMID: 35751041 Free PMC article.
References
-
- Kopke M., Mihalcea C., Liew F., Tizard J., Ali M., Conolly J., Al-Sinawi B., Simpson S. 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011;77:5467–5475. doi: 10.1128/AEM.00355-11. - DOI - PMC - PubMed
-
- Dgk A., Swy A., Mk A., Jkk B., Yubc D., Mko A. Improved 2,3-butanediol yield and productivity from lignocellulose biomass hydrolysate in metabolically engineered Enterobacter Aerogenes. Bioresour. Technol. 2020;309:123386. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

