Pentacyclic Triterpenoids Isolated from Celastraceae: A Focus in the 13C-NMR Data
- PMID: 35164224
- PMCID: PMC8838773
- DOI: 10.3390/molecules27030959
Pentacyclic Triterpenoids Isolated from Celastraceae: A Focus in the 13C-NMR Data
Abstract
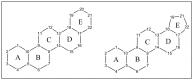
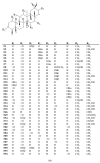
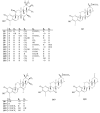
The Celastraceae family comprises about 96 genera and more than 1.350 species, occurring mainly in tropical and subtropical regions of the world. The species of this family stand out as important plant sources of triterpenes, both in terms of abundance and structural diversity. Triterpenoids found in Celastraceae species display mainly lupane, ursane, oleanane, and friedelane skeletons, exhibiting a wide range of biological activities such as antiviral, antimicrobial, analgesic, anti-inflammatory, and cytotoxic against various tumor cell lines. This review aimed to document all triterpenes isolated from different botanical parts of species of the Celastraceae family covering 2001 to 2021. Furthermore, a compilation of their 13C-NMR data was carried out to help characterize compounds in future investigations. A total of 504 pentacyclic triterpenes were compiled and distinguished as 29 aromatic, 50 dimers, 103 friedelanes, 89 lupanes, 102 oleananes, 22 quinonemethides, 88 ursanes and 21 classified as others.
Keywords: 13C-NMR; Celastraceae; quinonemethide; triterpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




















References
-
- Christenhusz M.J.M., Byng J.W. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;216:201–217. doi: 10.11646/phytotaxa.261.3.1. - DOI
-
- Rodrigues A.C.B.C., Oliveira F.P., Dias R.B., Sales C.B.S., Rocha C.A.G., Soares M.B.P., Costa E.V., Silva F.M.A., Rocha W.C., Koolen H.H.F., et al. In vitro and in vivo anti-leukemia activity of the stem bark of Salacia impressifolia (Miers) A. C. Smith (Celastraceae) J. Ethnopharmacol. 2019;231:516–524. doi: 10.1016/j.jep.2018.11.008. - DOI - PubMed
-
- Menezes L.D., Gomes G.O., Antônio J., Da P., Neto S., Laundry M.M., Ferreira V.M., Valero S.M. Evaluation of anti-inflammatory and antinociceptive activities of the Austroplenckia populnea extract in topical formulations. Afr. J. Pharm. Pharmacol. 2014;8:1180–1185. doi: 10.5897/AJPP2014.4167. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

